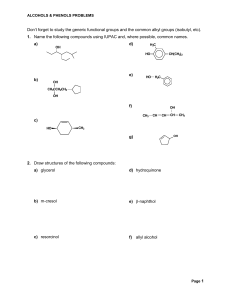
Don`t forget to study the generic functional groups and the common
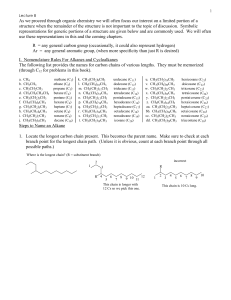
... 11. Write equations to show how the following transformation can be carried out. More than one step may be necessary. There are marks assigned for each intermediate product (not charged transition states) and there are marks for the reagents used; so list them all. No marks are assigned for mechanis ...
... 11. Write equations to show how the following transformation can be carried out. More than one step may be necessary. There are marks assigned for each intermediate product (not charged transition states) and there are marks for the reagents used; so list them all. No marks are assigned for mechanis ...
Three-dimensional Arrangement Of Atoms
... Addition of HBr to a chiral alkene: reactions of a chiral reactant with an achiral reagent gives diastereomeric products which may or may not be formed in equal amounts. The intermediate carbocation is asymmetric, therefore attack of Br- from the top or bottom faces may not be equally probable. The ...
... Addition of HBr to a chiral alkene: reactions of a chiral reactant with an achiral reagent gives diastereomeric products which may or may not be formed in equal amounts. The intermediate carbocation is asymmetric, therefore attack of Br- from the top or bottom faces may not be equally probable. The ...
coordination of some monodentate and hybrid multident ate
... ligand exhibit fluxional behaviour in solution, where the phosphine is interchanging between mono- and bi-dentate modes of coordination. In contrast, fluxionality has not been observed in the case of the ortho-vinyl substituted ligand. A set of platinum(II)-, osmium(II)- and ruthenium(II)-fluoride t ...
... ligand exhibit fluxional behaviour in solution, where the phosphine is interchanging between mono- and bi-dentate modes of coordination. In contrast, fluxionality has not been observed in the case of the ortho-vinyl substituted ligand. A set of platinum(II)-, osmium(II)- and ruthenium(II)-fluoride t ...
What is Organic Chemistry?
... Antibonding orbital..node between nuclei (zero electron density) σ bond is cylindrically symmetrical Aufbau principle...fill lowest energy orbitals first Hund's Rule...place one electron in each degenerate orbital first, then pair up Pauli Exclusion Principle...two electrons in the same orbital must ...
... Antibonding orbital..node between nuclei (zero electron density) σ bond is cylindrically symmetrical Aufbau principle...fill lowest energy orbitals first Hund's Rule...place one electron in each degenerate orbital first, then pair up Pauli Exclusion Principle...two electrons in the same orbital must ...
Elimination Reactions
... neutral conditions with polar solvents, such as water, ethyl alcohol or acetic acid. • E1 reactions can also occur with strong bases, but only at low concentration, about 0.01 to 0.1 M or below. • E2 reactions require strong base in high concentration, about 1 M or above. WWU -- Chemistry ...
... neutral conditions with polar solvents, such as water, ethyl alcohol or acetic acid. • E1 reactions can also occur with strong bases, but only at low concentration, about 0.01 to 0.1 M or below. • E2 reactions require strong base in high concentration, about 1 M or above. WWU -- Chemistry ...
Chapter 12 Organic Compounds with Oxygen and Sulfur
... Stereoisomers have identical molecular formulas, but they are not structural isomers. In stereoisomers, the atoms are bonded in the same sequence but differ in the way they are arranged in space. When stereoisomers have mirror images that are different, they are said to have “handedness.” ...
... Stereoisomers have identical molecular formulas, but they are not structural isomers. In stereoisomers, the atoms are bonded in the same sequence but differ in the way they are arranged in space. When stereoisomers have mirror images that are different, they are said to have “handedness.” ...
R - Evans - Harvard University
... reaction^.^^*^.^ In conjunction with our interests in amino acid derived natural products, we anticipated that these enolates might be attractive precursors to enantiomerically pure a-amino acids if effective electrophilic aminating agents could be developed. The simple strategy that was envisioned ...
... reaction^.^^*^.^ In conjunction with our interests in amino acid derived natural products, we anticipated that these enolates might be attractive precursors to enantiomerically pure a-amino acids if effective electrophilic aminating agents could be developed. The simple strategy that was envisioned ...
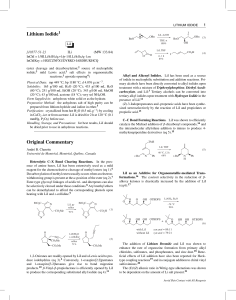
Lithium Bromide Original Commentary
... (90 ◦ C); 73 g/100 mL EtOH (40 ◦ C); 8 g/100 mL MeOH; sol ...
... (90 ◦ C); 73 g/100 mL EtOH (40 ◦ C); 8 g/100 mL MeOH; sol ...
Metallocene Organoactinide Complexes
... providing for greater flexibility in affecting chemical transformations. During the 1960s, the main technological interest in organoactinide chemistry lay in its potential for application in isotope separation processes [5], but at present the advancement of sophistication has put impetus on the inte ...
... providing for greater flexibility in affecting chemical transformations. During the 1960s, the main technological interest in organoactinide chemistry lay in its potential for application in isotope separation processes [5], but at present the advancement of sophistication has put impetus on the inte ...
Aldehydes and ketones
... 1. Oxidation – Tollens Test - Benedicts Test 2. Reduction – Hydrogen addition – NaBH4 reagent 3. Addition of Alcohols – hemiacetal/acetal and tautomerism ...
... 1. Oxidation – Tollens Test - Benedicts Test 2. Reduction – Hydrogen addition – NaBH4 reagent 3. Addition of Alcohols – hemiacetal/acetal and tautomerism ...
Chapter 17 Amines
... 756; M. N. Khan, J. Org. Chem. 61, 8063 (1996). Stereoselectivity: A. Kubo et al., Tetrahedron Letters 37, ...
... 756; M. N. Khan, J. Org. Chem. 61, 8063 (1996). Stereoselectivity: A. Kubo et al., Tetrahedron Letters 37, ...
1H-Imidazol-4(5H)-ones and thiazol-4(5H)
... character of the β-aryl substituent of nitroalkene 5 (compounds 6–8). Different substituents either at the nitrogen atom of the starting imidazolone (compounds 9–11) or at the 5-position (compound 12) of the ring are also well tolerated. Reactions with β-alkyl nitroolefins in the presence of C1 (Sch ...
... character of the β-aryl substituent of nitroalkene 5 (compounds 6–8). Different substituents either at the nitrogen atom of the starting imidazolone (compounds 9–11) or at the 5-position (compound 12) of the ring are also well tolerated. Reactions with β-alkyl nitroolefins in the presence of C1 (Sch ...
中国科学技术大学有机合成讲义-2
... turnover. It is proposed to avoid the formation of insoluble Ru-carboxylate complexes and ...
... turnover. It is proposed to avoid the formation of insoluble Ru-carboxylate complexes and ...
Lithium Iodide Original Commentary - Groupe Charette
... LiI as an Additive for Organometallic-mediated Transformations. Diastereoselectivity in the cyclization of 5-hexenyllithiums was shown to be influenced by LiI.34 As an additive in the reduction of α,β-unsaturated ketones by Bu2 SnH2 /Bu2 SnF2 , LiI has a dramatic effect on the selectivity for 1,2- ve ...
... LiI as an Additive for Organometallic-mediated Transformations. Diastereoselectivity in the cyclization of 5-hexenyllithiums was shown to be influenced by LiI.34 As an additive in the reduction of α,β-unsaturated ketones by Bu2 SnH2 /Bu2 SnF2 , LiI has a dramatic effect on the selectivity for 1,2- ve ...
Oxo-molybdenum(VI) - Repositório da Universidade Nova de Lisboa
... triggers its capability to catalyze different kind of processes such as carbon-carbon bond formation, olefin metathesis or alkene epoxidation among the most relevant transformations. ...
... triggers its capability to catalyze different kind of processes such as carbon-carbon bond formation, olefin metathesis or alkene epoxidation among the most relevant transformations. ...
haloalkanes and arenes
... Freon-12 (dichlorodifluoromethane, CF2Cl2) is commonly known as CFC. It is used as a refrigerant in refrigerators and air conditioners. It is also used in aerosol spray propellants such as body sprays, hair sprays, etc. However, it damages the ozone layer. Hence, its manufacture was banned in the Un ...
... Freon-12 (dichlorodifluoromethane, CF2Cl2) is commonly known as CFC. It is used as a refrigerant in refrigerators and air conditioners. It is also used in aerosol spray propellants such as body sprays, hair sprays, etc. However, it damages the ozone layer. Hence, its manufacture was banned in the Un ...
Ion Exchange Resins: Catalyst Recovery and Recycle
... simple filtration or decantation. This is not the case for other powdered materials often used as catalysts support (e.g., silica, zeolites, carbon, etc.). When the particle size is about 1 µm or less, they might not settle in the solution within a short time, and it would be very difficult to colle ...
... simple filtration or decantation. This is not the case for other powdered materials often used as catalysts support (e.g., silica, zeolites, carbon, etc.). When the particle size is about 1 µm or less, they might not settle in the solution within a short time, and it would be very difficult to colle ...
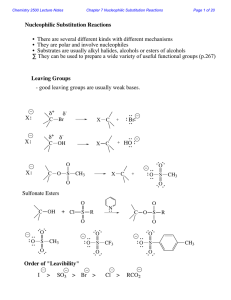
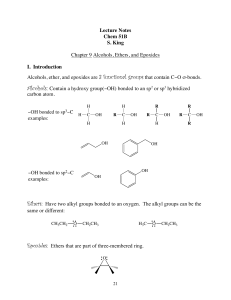
Lecture Notes Chem 51B S. King Chapter 9 Alcohols, Ethers, and
... X. Reactions of Epoxides Ethers in which the oxygen atom is incorporated into a 3-membered ring are called epoxides (oxiranes.) Even though an epoxide and an ether have the same L.G., epoxides are much more reactive than ethers because the strain of the 3-membered ring is relieved when the ring open ...
... X. Reactions of Epoxides Ethers in which the oxygen atom is incorporated into a 3-membered ring are called epoxides (oxiranes.) Even though an epoxide and an ether have the same L.G., epoxides are much more reactive than ethers because the strain of the 3-membered ring is relieved when the ring open ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.