Sample Exam #2 Answer Key
... bonding properties while thiols are incapable of hydrogen bonding. Consequently, alcohols have comparatively higher melting points, boiling points and densities than thiols. Finally, alcohols are much more water soluble than thiols due to their greater polarity and hydrogen bonding. 2) Compare the m ...
... bonding properties while thiols are incapable of hydrogen bonding. Consequently, alcohols have comparatively higher melting points, boiling points and densities than thiols. Finally, alcohols are much more water soluble than thiols due to their greater polarity and hydrogen bonding. 2) Compare the m ...
Lecture - Ch 24
... • Electrophilic aromatic substitution – Amino substituents are strongly activating and ortho- and para-directing groups in electrophilic aromatic substitution reactions – Reactions are controlled by conversion to amide ...
... • Electrophilic aromatic substitution – Amino substituents are strongly activating and ortho- and para-directing groups in electrophilic aromatic substitution reactions – Reactions are controlled by conversion to amide ...
Handout VI
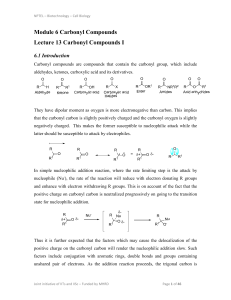
... act as a nucleophile and add to the carbonyl group to yield a radical anion often as ion pair with the metal cation. The blue colour generated by the aromatic ketones on reaction with sodium in the absence of air is an example of formation of such a radical. ...
... act as a nucleophile and add to the carbonyl group to yield a radical anion often as ion pair with the metal cation. The blue colour generated by the aromatic ketones on reaction with sodium in the absence of air is an example of formation of such a radical. ...
Document
... Functional Groups • Besides our basic hydrocarbon classes, we can add other elements/ions, groups of elements to an organic structure = Functional Groups • R = radical or, in this case, represents C • Alcohols (R-OH), ethers (R-O-R), carboxylic acids (R-COOH), aldehydes (R-COH) – what are the other ...
... Functional Groups • Besides our basic hydrocarbon classes, we can add other elements/ions, groups of elements to an organic structure = Functional Groups • R = radical or, in this case, represents C • Alcohols (R-OH), ethers (R-O-R), carboxylic acids (R-COOH), aldehydes (R-COH) – what are the other ...
Alcohols, Phenols, and Thiols
... right-hand side of the equation is water with a pKa of about 16. Thus, the equilibrium lies to the side of water, the weaker acid. In part d, we need to compare the pKas of cyclopentanol and water. Table 7.2 suggests that the pKa of cyclopentanol might be very close to that of water, so one might ex ...
... right-hand side of the equation is water with a pKa of about 16. Thus, the equilibrium lies to the side of water, the weaker acid. In part d, we need to compare the pKas of cyclopentanol and water. Table 7.2 suggests that the pKa of cyclopentanol might be very close to that of water, so one might ex ...
Document
... alcohol with either 85% H3PO4 or concentrated H2SO4 • 1° alcohols are the most difficult to dehydrate and require temperatures as high as 180°C • 2° alcohols undergo acid-catalyzed dehydration at somewhat lower temperatures • 3° alcohols generally undergo acid-catalyzed dehydration at temperatures o ...
... alcohol with either 85% H3PO4 or concentrated H2SO4 • 1° alcohols are the most difficult to dehydrate and require temperatures as high as 180°C • 2° alcohols undergo acid-catalyzed dehydration at somewhat lower temperatures • 3° alcohols generally undergo acid-catalyzed dehydration at temperatures o ...
Organic Chemistry II with Dr Roche
... Since ethers are relatively unreactive and are strongly polar (due to the lone pairs on the oxygen), they are commonly used as solvents for organic reactions. (Diethyl ether and THF, the Grignard reaction). Ethers will often form complexes with molecules that have vacant orbitals, enabling ‘unstable ...
... Since ethers are relatively unreactive and are strongly polar (due to the lone pairs on the oxygen), they are commonly used as solvents for organic reactions. (Diethyl ether and THF, the Grignard reaction). Ethers will often form complexes with molecules that have vacant orbitals, enabling ‘unstable ...
phenols - Gneet`s
... Sodium salt of aryl sulphonic acids on fusion with sodium hydroxide at 300-350oC yield phenol ...
... Sodium salt of aryl sulphonic acids on fusion with sodium hydroxide at 300-350oC yield phenol ...
Learning Guide for Chapter 23: Amines
... mixture is extracted with dilute aqueous acid, which converts the amine to the amine salt which is soluble in the water layer. After the separation, treatment with dilute base restores the amine. ...
... mixture is extracted with dilute aqueous acid, which converts the amine to the amine salt which is soluble in the water layer. After the separation, treatment with dilute base restores the amine. ...
Document
... the lowest number. Apply all of the usual rules of nomenclature. • With cyclic ketones, numbering always begins at the carbonyl carbon, but the “1” is usually omitted from the name. The ring is then numbered clockwise or counterclockwise to give the first substituent the lower number. ...
... the lowest number. Apply all of the usual rules of nomenclature. • With cyclic ketones, numbering always begins at the carbonyl carbon, but the “1” is usually omitted from the name. The ring is then numbered clockwise or counterclockwise to give the first substituent the lower number. ...
HMDS - Sigma
... It is a popular choice for silylating acids, alcohols, amines, and phenols. HMDS can be used alone, but derivatization usually will proceed faster with a catalyst. HMDS also is useful for conditioning chromatography columns and for deactivating glassware or silica. ...
... It is a popular choice for silylating acids, alcohols, amines, and phenols. HMDS can be used alone, but derivatization usually will proceed faster with a catalyst. HMDS also is useful for conditioning chromatography columns and for deactivating glassware or silica. ...
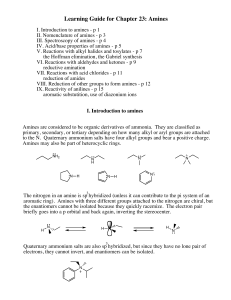
Amines
... atom, the nitrogen bears a positive charge name the compound as a salt replace the ending -amine (or aniline or pyridine or the like) by -ammonium (or anilinium or pyridinium or the like) and add the name of the anion ...
... atom, the nitrogen bears a positive charge name the compound as a salt replace the ending -amine (or aniline or pyridine or the like) by -ammonium (or anilinium or pyridinium or the like) and add the name of the anion ...
CH 3 - bYTEBoss
... • Organic molecules exhibit three different types of hybridization at the carbon center: – sp3 hybridized carbons for tetrahedral geometries; – sp2 hybridized carbons for trigonal planar geometries; and – sp hybridized carbons for linear geometries. ...
... • Organic molecules exhibit three different types of hybridization at the carbon center: – sp3 hybridized carbons for tetrahedral geometries; – sp2 hybridized carbons for trigonal planar geometries; and – sp hybridized carbons for linear geometries. ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... transformation [1]. Diverse reagents and reaction conditions have been developed for this purpose. Conversion of aromatic amines to corresponding acetamides is also well documented [2]. Reduction of nitro group and acetylation of the resulting amine are often carried out in succession to attenuate t ...
... transformation [1]. Diverse reagents and reaction conditions have been developed for this purpose. Conversion of aromatic amines to corresponding acetamides is also well documented [2]. Reduction of nitro group and acetylation of the resulting amine are often carried out in succession to attenuate t ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.