Basic Organic Nomenclature and Functional Groups Notes
... Names are changed to reflect the functional group(s) Cyclo-hydrocarbons ...
... Names are changed to reflect the functional group(s) Cyclo-hydrocarbons ...
Introduction to Organic Chemistry Notes Sheet
... What happens if the alkane has more than one substituent? In this case, the rules above are followed, and the carbons on the longest chain are numbered to give the lowest number possible to one of the substituent. The substituents are then all named in the prefix (e.g. 2-ethyl,3-methyl). If more tha ...
... What happens if the alkane has more than one substituent? In this case, the rules above are followed, and the carbons on the longest chain are numbered to give the lowest number possible to one of the substituent. The substituents are then all named in the prefix (e.g. 2-ethyl,3-methyl). If more tha ...
Alcohols, Ethers, and Epoxides
... Don’t forget about using tosylates in substitution and elimination reactions as well. In many cases, they are better than their halide counterparts. Reaction of ethers and epoxides: ...
... Don’t forget about using tosylates in substitution and elimination reactions as well. In many cases, they are better than their halide counterparts. Reaction of ethers and epoxides: ...
study note 3 33
... You should be familiar with the rules for naming compounds that contain functional groups (handout) Give structures for these common names Methyl alcohol CH3–OH Ethyl alcohol CH3CH2–OH OH Isopropyl alcohol H3C ...
... You should be familiar with the rules for naming compounds that contain functional groups (handout) Give structures for these common names Methyl alcohol CH3–OH Ethyl alcohol CH3CH2–OH OH Isopropyl alcohol H3C ...
Chapter 12 - Alcohols from Carbonyl Compounds1
... - When a Grignard reagent adds to the carbonyl group of an ester, the initial product breaks down to a ketone. Then, the ketone reacts with the excess Grignard reagent and then goes through hydrolysis to form a tertiary alcohol with two identical alkyl groups ...
... - When a Grignard reagent adds to the carbonyl group of an ester, the initial product breaks down to a ketone. Then, the ketone reacts with the excess Grignard reagent and then goes through hydrolysis to form a tertiary alcohol with two identical alkyl groups ...
Introduction and Alk.. - hrsbstaff.ednet.ns.ca
... atoms, thus containing only single bonds Unsaturated: organic compounds in which the carbon atoms do not have the maximum number of hydrogen atoms and thus have double or triple bonds ...
... atoms, thus containing only single bonds Unsaturated: organic compounds in which the carbon atoms do not have the maximum number of hydrogen atoms and thus have double or triple bonds ...
CH 3502 4500
... 16. Discuss the mechanism of cleavage of ethers by HI. 17. Explain Williamson’s synthesis of ethers. 18. Discuss Norrish type-I reaction. 19. Discuss the mechanism of Wittig reaction and its uses in organic synthesis. 20. Explain Wolf-Kishner reduction with its mechanism. 21. Give any two methods o ...
... 16. Discuss the mechanism of cleavage of ethers by HI. 17. Explain Williamson’s synthesis of ethers. 18. Discuss Norrish type-I reaction. 19. Discuss the mechanism of Wittig reaction and its uses in organic synthesis. 20. Explain Wolf-Kishner reduction with its mechanism. 21. Give any two methods o ...
Document
... halogens > O, S >> N, C. This cleavage is heterolytic, and requires that the charge site migrates. This means that this reaction is less favoured than radical-site reactions. 4) Decomposition of cyclic structures To produce a fragment from a ring, 2 bonds must be broken. If only one bond is cleaved, ...
... halogens > O, S >> N, C. This cleavage is heterolytic, and requires that the charge site migrates. This means that this reaction is less favoured than radical-site reactions. 4) Decomposition of cyclic structures To produce a fragment from a ring, 2 bonds must be broken. If only one bond is cleaved, ...
OME General Chemistry
... π-bonding forces 2 CH2-groups in ethylene to bond so as to give a planar structure Geometric isomers: atoms are joined to one another in the same way but differ because some atoms occupy different relative positions in space. ...
... π-bonding forces 2 CH2-groups in ethylene to bond so as to give a planar structure Geometric isomers: atoms are joined to one another in the same way but differ because some atoms occupy different relative positions in space. ...
Non-Heme Iron Catalyzed Oxidation of Alkanes to Alcohols via
... The ability of the binuclear non-heme ferrous complex Fe22+(H2Hbamb)2(NMeIm)2 (H2Hbamb: 2,3-bis(2-hydroxybenzamido)2,3-dimethylbutane) to react with oxygen atom donor molecules, peroxides or peracids and catalyze the hydroxylation of alkanes and arenes will be discussed. Mechanistic studies demonstr ...
... The ability of the binuclear non-heme ferrous complex Fe22+(H2Hbamb)2(NMeIm)2 (H2Hbamb: 2,3-bis(2-hydroxybenzamido)2,3-dimethylbutane) to react with oxygen atom donor molecules, peroxides or peracids and catalyze the hydroxylation of alkanes and arenes will be discussed. Mechanistic studies demonstr ...
Organic Chemistry: Introduction
... • To get a carboxylic acid, the aldehyde has to remain in the solution with the oxidizing agent for a longer amount of time. Instead of distillation, a reflux column is used. • Refluxing is a technique that involves the cyclic evaporation and condensation of a volatile reaction mixture, preserving t ...
... • To get a carboxylic acid, the aldehyde has to remain in the solution with the oxidizing agent for a longer amount of time. Instead of distillation, a reflux column is used. • Refluxing is a technique that involves the cyclic evaporation and condensation of a volatile reaction mixture, preserving t ...
Click for Section 2.9 notes
... • Alkanes contain only C and H and are called hydrocarbons • The names of alkanes all end in the suffix –ane • Alkanes are named according to the number of C atoms in their backbone chain: ...
... • Alkanes contain only C and H and are called hydrocarbons • The names of alkanes all end in the suffix –ane • Alkanes are named according to the number of C atoms in their backbone chain: ...
review sheet plus practice problems
... Questions that may appear on the exam: What is the name for this alkyl halide / alcohol / ether? Is an alcohol 1°, 2°, or 3°? What are the products of free radical halogenation of an alkane (ex: Cl2/uv light)? Give the chain mechanism for free radical halogenation. What is the selectivity for bromin ...
... Questions that may appear on the exam: What is the name for this alkyl halide / alcohol / ether? Is an alcohol 1°, 2°, or 3°? What are the products of free radical halogenation of an alkane (ex: Cl2/uv light)? Give the chain mechanism for free radical halogenation. What is the selectivity for bromin ...
III. ORGANIC CHEMISTRY Reactions
... always take note of the catalysts and conditions required for a reaction to occur; for example, Br2 and Cl2 are easily disrupted in the presence of double and triple bonds – addition reactions with these occur readily; H2 and H2O are much more stable and therefore reactions involving these still nee ...
... always take note of the catalysts and conditions required for a reaction to occur; for example, Br2 and Cl2 are easily disrupted in the presence of double and triple bonds – addition reactions with these occur readily; H2 and H2O are much more stable and therefore reactions involving these still nee ...
Regents Unit 15b: Halides, Alcohols, & Ethers
... Uses of Alkyl Halides • Cleaners & solvents • Teflon & PVC’s are alkyl halides. • Refrigerants. (used to be chlorofluorocarbons. Now hydrofluorocarbons.) • Starting materials in many reactions. ...
... Uses of Alkyl Halides • Cleaners & solvents • Teflon & PVC’s are alkyl halides. • Refrigerants. (used to be chlorofluorocarbons. Now hydrofluorocarbons.) • Starting materials in many reactions. ...
Chapter 6: Alkynes, reactions of alkynes, and multistep synthesis
... b. can stop only if using “poison” catalyst (Lindlar catalyst) c. get cis alkene for syn addition with Lindlar d. to get trans, use Na or Li in liquid ammonia (-78ºC) e. this is radical addition ...
... b. can stop only if using “poison” catalyst (Lindlar catalyst) c. get cis alkene for syn addition with Lindlar d. to get trans, use Na or Li in liquid ammonia (-78ºC) e. this is radical addition ...
Chapter 10 for 301
... There cannot be any acidic protons in the solvent, as the Grignard is such a strong base. There cannot be any pi bonds in the solvent as those are sites of reactivity that the Grignard will attack. From here on, I will use Grignard to refer to both Grignard reagents and organolithiums, as they ...
... There cannot be any acidic protons in the solvent, as the Grignard is such a strong base. There cannot be any pi bonds in the solvent as those are sites of reactivity that the Grignard will attack. From here on, I will use Grignard to refer to both Grignard reagents and organolithiums, as they ...
Hydrocarbon Derivatives:
... • Dipole-dipole: intermediate strength – molecule has atoms with different electronegativities – atoms not arranged symmetrically ...
... • Dipole-dipole: intermediate strength – molecule has atoms with different electronegativities – atoms not arranged symmetrically ...
File - the prayas tutorial
... in aq. solution, OH– ions are highly solvated (hydrated). This solution reduces the basic character of OH– ions which fail to abstract a hydrogen from the -carbon of the alkyl halide to form an alkene. However an alcoholic solution of KOH contains alkoxide (RO–) ions which being a much stronger bas ...
... in aq. solution, OH– ions are highly solvated (hydrated). This solution reduces the basic character of OH– ions which fail to abstract a hydrogen from the -carbon of the alkyl halide to form an alkene. However an alcoholic solution of KOH contains alkoxide (RO–) ions which being a much stronger bas ...
Alcohol Worksheet Key
... 1 and 3 exhibit H-bonding, but 3 has a higher molecular weight. It has the highest boiling point. 2 has the lowest boiling point because it doesn’t exhibit H-bonding. ...
... 1 and 3 exhibit H-bonding, but 3 has a higher molecular weight. It has the highest boiling point. 2 has the lowest boiling point because it doesn’t exhibit H-bonding. ...
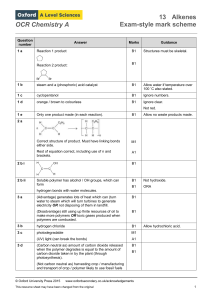
OCR Chemistry A Question number Answer Marks Guidance 1 a
... (In order to have cis or trans isomers) each C atom of the CC double bond must have two different substituent groups and one of those groups must be ...
... (In order to have cis or trans isomers) each C atom of the CC double bond must have two different substituent groups and one of those groups must be ...
Development of New Organic Reactions by Exploiting Sulfur
... The structures of target molecules in organic synthesis are becoming more complicated, and better functional compatibility and higher selectivity are required for the efficient synthesis of complex molecules. However, these requirements are not always fulfilled with conventional organic reactions, e ...
... The structures of target molecules in organic synthesis are becoming more complicated, and better functional compatibility and higher selectivity are required for the efficient synthesis of complex molecules. However, these requirements are not always fulfilled with conventional organic reactions, e ...
Structure and Shape - Chapter 12 Valence Shell Electron Pair
... isomers - molecules with the same molecular formula but different arrangements of atoms. Examples: pentane, 2-methylbutane 2- methylbutane,, 2,2-dimethylpropane 2,2-dimethylpropane Each isomer is different, having its own physical and chemical properties. - e.g. boiling points and melting points ...
... isomers - molecules with the same molecular formula but different arrangements of atoms. Examples: pentane, 2-methylbutane 2- methylbutane,, 2,2-dimethylpropane 2,2-dimethylpropane Each isomer is different, having its own physical and chemical properties. - e.g. boiling points and melting points ...
Chapter 26 Functional Groups and Organic Reactions
... Alcohols - a class of organic compounds with an -OH group – The -OH functional group in alcohols is called a “hydroxyl” group; thus ROH can represent the formula, where “R” is an alkyl group How is this different from the hydroxide ion? (covalently bonded to the carbonnot ionically as in hydroxide ...
... Alcohols - a class of organic compounds with an -OH group – The -OH functional group in alcohols is called a “hydroxyl” group; thus ROH can represent the formula, where “R” is an alkyl group How is this different from the hydroxide ion? (covalently bonded to the carbonnot ionically as in hydroxide ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.