Asbtracts of Talks at ICEC 2014 - Association of Chemistry Teachers
... The educational import of History and Philosophy of Science (HPS) is a growing area of science education research globally. HPS can be a useful pedagogic resource to improve science learning in different ways. Carefully prepared historical vignettes on suitable topics can help students confront thei ...
... The educational import of History and Philosophy of Science (HPS) is a growing area of science education research globally. HPS can be a useful pedagogic resource to improve science learning in different ways. Carefully prepared historical vignettes on suitable topics can help students confront thei ...
Chapter 3 Molecules, Compounds, and Chemical Equations
... • Inorganic compounds are very difficult to decompose, but can be synthesized. ...
... • Inorganic compounds are very difficult to decompose, but can be synthesized. ...
8.P.1.1 Warm-Up Questions for Website
... B.It can be formed through a physical reaction. C.It can be changed into simpler substances through a physical change. D.It is a pure substance containing elements that are chemically combined. ...
... B.It can be formed through a physical reaction. C.It can be changed into simpler substances through a physical change. D.It is a pure substance containing elements that are chemically combined. ...
Revision Y12 Chemistry PLC
... (a) ionic bonding as electrostatic attraction between positive and negative ions, and the construction of 'dot-and-cross' diagrams (b) explanation of the solid structures of giant ionic lattices, resulting from oppositely charged ions strongly attracted in all directions e.g. NaCl (c) explanation of ...
... (a) ionic bonding as electrostatic attraction between positive and negative ions, and the construction of 'dot-and-cross' diagrams (b) explanation of the solid structures of giant ionic lattices, resulting from oppositely charged ions strongly attracted in all directions e.g. NaCl (c) explanation of ...
Atoms and Molecules
... This is an optional, nongraded section. It will be one free response question on the AP Exam. For each set of reactants: state the type of reaction, write the formulas of the reactants and predict the products a. ...
... This is an optional, nongraded section. It will be one free response question on the AP Exam. For each set of reactants: state the type of reaction, write the formulas of the reactants and predict the products a. ...
Chemistry Nomenclature Notes
... -They become positively charged and are called cations. -The size of the positive charge is determined by the number of electrons lost. -The number of electrons lost is determined by the proximity to the nearest Noble gas. -Named by using the full metals name and adding ion at the end. Ex: Magnesium ...
... -They become positively charged and are called cations. -The size of the positive charge is determined by the number of electrons lost. -The number of electrons lost is determined by the proximity to the nearest Noble gas. -Named by using the full metals name and adding ion at the end. Ex: Magnesium ...
Molecules and Ions
... Ionic formulas ALWAYS have a ZERO net charge – i.e. the (+) and (-) ionic charges in ANY formula cancel. If the above rule is followed, the ionic compound must exist and is probably sitting on a shelf in the chemistry stock room! Task: Construct and name as many ionic compounds as possible from the ...
... Ionic formulas ALWAYS have a ZERO net charge – i.e. the (+) and (-) ionic charges in ANY formula cancel. If the above rule is followed, the ionic compound must exist and is probably sitting on a shelf in the chemistry stock room! Task: Construct and name as many ionic compounds as possible from the ...
Name: Northwest Vista College Chem 1311
... 17. What is the charge on the monatomic ion of nitrogen, the nitride ion? A) +2 B) +1 C) –1 D) –2 E) –3 18. Which of the elements listed below has the greatest atomic radius? A) B B) Al C) S D) P E) Si 19. Which of the following cations has the lowest Lattice energy with the same anion? A) Li+ B) N ...
... 17. What is the charge on the monatomic ion of nitrogen, the nitride ion? A) +2 B) +1 C) –1 D) –2 E) –3 18. Which of the elements listed below has the greatest atomic radius? A) B B) Al C) S D) P E) Si 19. Which of the following cations has the lowest Lattice energy with the same anion? A) Li+ B) N ...
Chemistry I Syllabus 2011-2012
... inorganic, kinetic energy, law of conservation of mass, light energy, luster, malleability, melting point, metal, metalloid, molecule, nonmetal, organic, phase change, physical properties, potential energy, pure substance, reactivity with air (oxidation), solute, solution, solvent, strength, sub-let ...
... inorganic, kinetic energy, law of conservation of mass, light energy, luster, malleability, melting point, metal, metalloid, molecule, nonmetal, organic, phase change, physical properties, potential energy, pure substance, reactivity with air (oxidation), solute, solution, solvent, strength, sub-let ...
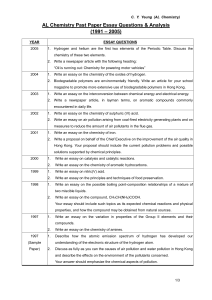
AL Chemistry Past paper essay questions
... Write an essay on the compound, CH 3CH(NH2)COOH. Your essay should include such topics as its expected chemical reactions and physical properties, and how the compound may be obtained from natural sources. ...
... Write an essay on the compound, CH 3CH(NH2)COOH. Your essay should include such topics as its expected chemical reactions and physical properties, and how the compound may be obtained from natural sources. ...
8F Compounds and Mixtures
... 3. The substances in a mixture are just mixed, not chemically joined, and so it is usually quite easy to separate the ingredients (e.g. it is easy to get salt from sea water). ...
... 3. The substances in a mixture are just mixed, not chemically joined, and so it is usually quite easy to separate the ingredients (e.g. it is easy to get salt from sea water). ...
Summer Assignment
... 5. Oxygen has an oxidation number of –2 unless it is combined with F, when it is +2, or it is in a peroxide, when it is –1. ...
... 5. Oxygen has an oxidation number of –2 unless it is combined with F, when it is +2, or it is in a peroxide, when it is –1. ...
AP_chemistry_Summer_Assignment_2014
... c.Phosphorus in PO43d.Manganese in MnO4269.Which of the following statements are always true? Never true? Not always true? a.A compound wit the molecular formula C6H6 has the same simplest formula. b.The mass percent of copper in CuO is less than in Cu2O. c.The limiting reactant is the one present i ...
... c.Phosphorus in PO43d.Manganese in MnO4269.Which of the following statements are always true? Never true? Not always true? a.A compound wit the molecular formula C6H6 has the same simplest formula. b.The mass percent of copper in CuO is less than in Cu2O. c.The limiting reactant is the one present i ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.