BS Chemistry - Government College University Faisalabad
... Relation of entropy and energy with equilibrium constant and their dependence on temperature. Clausius-Clapeyron equation. Chemical potential. Partial molar quantities. Laws of thermodynamics and their applications. Thermodynamic functions internal energy, enthalpy, entropy and free energy. Relation ...
... Relation of entropy and energy with equilibrium constant and their dependence on temperature. Clausius-Clapeyron equation. Chemical potential. Partial molar quantities. Laws of thermodynamics and their applications. Thermodynamic functions internal energy, enthalpy, entropy and free energy. Relation ...
Chemistry Midterm Review 2006
... 1. Transition elements are in the __________ block and the inner transitions are in the ________ block. 2. Group 1A and 2A are in the ___________ block and groups 3A to 8A are in the __________ block. 3. a. What is an atomic orbital? b. What shape is the s sublevel? c. The shape of the p sublevel? d ...
... 1. Transition elements are in the __________ block and the inner transitions are in the ________ block. 2. Group 1A and 2A are in the ___________ block and groups 3A to 8A are in the __________ block. 3. a. What is an atomic orbital? b. What shape is the s sublevel? c. The shape of the p sublevel? d ...
Analytical Chemistry - University of Delhi
... B.Sc Analytical Chemistry Analytical Chemistry is an applied, experimental field of science and is based not only on chemistry, but also on physics, biology, information theory and many fields of technology. It is of fundamental importance not only to all branches of chemistry but also to all biolo ...
... B.Sc Analytical Chemistry Analytical Chemistry is an applied, experimental field of science and is based not only on chemistry, but also on physics, biology, information theory and many fields of technology. It is of fundamental importance not only to all branches of chemistry but also to all biolo ...
13.2 Chemical Formulas
... H2O, tells us that a water molecule is made of the elements hydrogen (H) and oxygen (O) and that it takes two atoms of hydrogen and one atom of oxygen to build the molecule. For sodium nitrate, NaNO3, the chemical formula tells us there are three elements in the compound: sodium (Na), nitrogen (N), ...
... H2O, tells us that a water molecule is made of the elements hydrogen (H) and oxygen (O) and that it takes two atoms of hydrogen and one atom of oxygen to build the molecule. For sodium nitrate, NaNO3, the chemical formula tells us there are three elements in the compound: sodium (Na), nitrogen (N), ...
Name ______Mr. Perfect_______________________________
... 1. If the n quantum number of an atomic orbital is equal to 4, what are the possible values of l ? What are the possible values of ml if the quantum number l is equal to 1? (5 pts) l ranges from 0 to n-1 ...
... 1. If the n quantum number of an atomic orbital is equal to 4, what are the possible values of l ? What are the possible values of ml if the quantum number l is equal to 1? (5 pts) l ranges from 0 to n-1 ...
No Slide Title - McMaster Chemistry
... There is no precipitate. However there is an obvious change in O.N. It is also an example of an OXIDATION-REDUCTION REACTION Zn goes from O.N. = 0 in the metal to O.N. = +2 in the chloride salt H goes from O.N. = +1 in HCl (aq) to O.N. = 0 in the elemental gas 1A03/1E03 Types of Reactions (2) ...
... There is no precipitate. However there is an obvious change in O.N. It is also an example of an OXIDATION-REDUCTION REACTION Zn goes from O.N. = 0 in the metal to O.N. = +2 in the chloride salt H goes from O.N. = +1 in HCl (aq) to O.N. = 0 in the elemental gas 1A03/1E03 Types of Reactions (2) ...
Gas-Forming reactions Reactions that form a
... But they end up with the same number of electrons they start with. Every atom, ion or polyatomic ion has a formal oxidation number associated with it. This value compares the number of protons in an atom (positive charge) and the number of electrons assigned to that atom (negative charge). In many c ...
... But they end up with the same number of electrons they start with. Every atom, ion or polyatomic ion has a formal oxidation number associated with it. This value compares the number of protons in an atom (positive charge) and the number of electrons assigned to that atom (negative charge). In many c ...
Unit 1 Powerpoint
... particular preference or point of view that is personal, rather than scientific. Science aims to be objective, but scientists are human, too. Sometimes scientific data can be misinterpreted or misapplied by scientists who want to prove a particular point. Recommendations made by scientists with pers ...
... particular preference or point of view that is personal, rather than scientific. Science aims to be objective, but scientists are human, too. Sometimes scientific data can be misinterpreted or misapplied by scientists who want to prove a particular point. Recommendations made by scientists with pers ...
Atoms, Molecules and Ions
... forms both Fe+ and Fe2+ ions, we need to use the Stock system and call the compound iron(II) nitrate. (b) The cation is Na+ and the anion is HPO42− (hydrogen phosphate). Because sodium only forms one type of ion (Na+), there is no need to use sodium(I) in the name. The compound is sodium hydrogen ph ...
... forms both Fe+ and Fe2+ ions, we need to use the Stock system and call the compound iron(II) nitrate. (b) The cation is Na+ and the anion is HPO42− (hydrogen phosphate). Because sodium only forms one type of ion (Na+), there is no need to use sodium(I) in the name. The compound is sodium hydrogen ph ...
Descriptive Chemistry of Elements d-Block
... configuration of a d-block element can be represented as (n+1)s2 ndm or (n+1)s1 ndm where n = 3, 4, 5 or 6 and m = 1, 2, 3, …..or 10. When you look at the Periodic Table, you can see, that there are four series (or rows) in the d-block. They are the 3d, 4d, 5d and 6d-series and are in the 4th, 5th, ...
... configuration of a d-block element can be represented as (n+1)s2 ndm or (n+1)s1 ndm where n = 3, 4, 5 or 6 and m = 1, 2, 3, …..or 10. When you look at the Periodic Table, you can see, that there are four series (or rows) in the d-block. They are the 3d, 4d, 5d and 6d-series and are in the 4th, 5th, ...
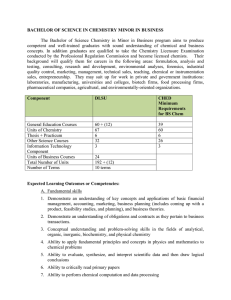
BACHELOR OF SCIENCE IN CHEMISTRY MINOR IN BUSINESS
... and its classifications; mass relationships in chemical reactions; the properties of gases, liquids, and solids; some concepts of thermochemistry; quantum theory and electronic behavior; periodic relationship of elements in the periodic table; chemical bonding; intramolecular forces; and solutions. ...
... and its classifications; mass relationships in chemical reactions; the properties of gases, liquids, and solids; some concepts of thermochemistry; quantum theory and electronic behavior; periodic relationship of elements in the periodic table; chemical bonding; intramolecular forces; and solutions. ...
B.Sc. Physical Sciences - Department of Computer Science
... spin, spin quantum number (s) and magnetic spin quantum number (ms). Electronic configurations of the atoms. Concept of exchange energy. Relative energies of atomic orbitals, Anomalous electronic configurations. Unit 2: Chemical Bonding and Molecular Structure Ionic Bonding : Energy considerations i ...
... spin, spin quantum number (s) and magnetic spin quantum number (ms). Electronic configurations of the atoms. Concept of exchange energy. Relative energies of atomic orbitals, Anomalous electronic configurations. Unit 2: Chemical Bonding and Molecular Structure Ionic Bonding : Energy considerations i ...
Writing Chemical Formulas and Chemical Reactions
... All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired number (coefficient ...
... All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired number (coefficient ...
B.Sc. Industrial Chemistry
... The course on B.Sc. Industrial Chemistry was introduced in the University of Delhi in 1984 and since then this course has undergone many changes and has become more comprehensive and relevant. The importance of industrial chemistry hardly needs any emphasis. It basically deals with the development, ...
... The course on B.Sc. Industrial Chemistry was introduced in the University of Delhi in 1984 and since then this course has undergone many changes and has become more comprehensive and relevant. The importance of industrial chemistry hardly needs any emphasis. It basically deals with the development, ...
Fall.2008.Week9.Lesson.1 - reich
... the left, BUT, I’m only speaking about the “types of molecules,” and I’m not invoking the coefficients. ...
... the left, BUT, I’m only speaking about the “types of molecules,” and I’m not invoking the coefficients. ...
Ch 11 Chemical Reactions
... What is a catalyst? A substance that speeds up a reaction, without being changed or used up by the reaction. Enzymes are biological catalysts in your body (proteins). ...
... What is a catalyst? A substance that speeds up a reaction, without being changed or used up by the reaction. Enzymes are biological catalysts in your body (proteins). ...
Basic Introduction of Computational Chemistry
... perform some test calculations on a small problem. Often methods that are not a natural fit have been extended, e.g. dispersion corrections in DFT Bottom line: Know your methods! ...
... perform some test calculations on a small problem. Often methods that are not a natural fit have been extended, e.g. dispersion corrections in DFT Bottom line: Know your methods! ...
Unit A Review Questions
... The zinc electrode is gaining mass because the copper ions are coming out of the solution and are being reduced by the zinc metal being oxidized. This would also account for the colour change in the copper nitrate solution. As the copper ions come out of the solution, the solution becomes a fainter ...
... The zinc electrode is gaining mass because the copper ions are coming out of the solution and are being reduced by the zinc metal being oxidized. This would also account for the colour change in the copper nitrate solution. As the copper ions come out of the solution, the solution becomes a fainter ...
word doc (perfect formatting)
... Questions 5-8 refer to the following descriptions of bonding in different types of solids. a) Lattice of positive and negative ions held together by electrostatic forces b) Closely packed lattice with delocalized electrons throughout giving ability to conduct electricity and permitting ductility c) ...
... Questions 5-8 refer to the following descriptions of bonding in different types of solids. a) Lattice of positive and negative ions held together by electrostatic forces b) Closely packed lattice with delocalized electrons throughout giving ability to conduct electricity and permitting ductility c) ...
The s-Block Elements - GCG-42
... BeCl2 is essentially covalent, with comparatively low m.pt. The lower members in group II form essentially ionic chlorides, with Mg having intermediate properties. ...
... BeCl2 is essentially covalent, with comparatively low m.pt. The lower members in group II form essentially ionic chlorides, with Mg having intermediate properties. ...
CHEMICAL REACTIONS
... with oxygen, often producing heat / light Burning of natural gas (methane) CH4 + 2O2 CO2 + 2 H2O TIP: If the reactants include a fuel & O2and the products include CO2 & H2O, it’s probably a combustion reaction ...
... with oxygen, often producing heat / light Burning of natural gas (methane) CH4 + 2O2 CO2 + 2 H2O TIP: If the reactants include a fuel & O2and the products include CO2 & H2O, it’s probably a combustion reaction ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.