CHEMISTRY The Central Science 9th Edition
... Are the elements which tend to lose one electron or more to form positive ions . Na → Na⁺ + e Ca → Ca⁺⁺ + 2e Metals form about 75% of elements. Metallic properties: increase when we move from right to left , and from top to the bottom in the periodic table. Metals have a characteristic luster and ge ...
... Are the elements which tend to lose one electron or more to form positive ions . Na → Na⁺ + e Ca → Ca⁺⁺ + 2e Metals form about 75% of elements. Metallic properties: increase when we move from right to left , and from top to the bottom in the periodic table. Metals have a characteristic luster and ge ...
School of Chemistry
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
AP Chemistry Summer Assignment - 2015
... Oxygen usually has an oxidation number of –2. Exceptions: In peroxides, such as H2O2, oxygen’s oxidation number is –1. In compounds with fluorine, such as OF2, oxygen’s oxidation number is +2. 5. Hydrogen has an oxidation number of +1 in all compounds containing elements that are more electronegat ...
... Oxygen usually has an oxidation number of –2. Exceptions: In peroxides, such as H2O2, oxygen’s oxidation number is –1. In compounds with fluorine, such as OF2, oxygen’s oxidation number is +2. 5. Hydrogen has an oxidation number of +1 in all compounds containing elements that are more electronegat ...
CVB101 – Lecture 3 Chemical Bonding • Chemical bonding
... Number of protons in the nucleus determines the chemical identity of the atom Chemical properties, most importantly, chemical reactivity is determined by the electrons, more precisely, electronic structure (number of eincluding their distribution around nucleus and their energies) – explained by ...
... Number of protons in the nucleus determines the chemical identity of the atom Chemical properties, most importantly, chemical reactivity is determined by the electrons, more precisely, electronic structure (number of eincluding their distribution around nucleus and their energies) – explained by ...
Review-Semester Final (Part I)
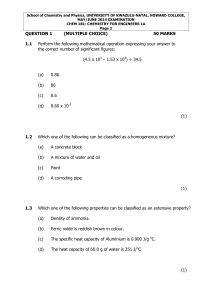
... b. Largest to smallest electronegativity:________________________________________ c. Largest to smallest ionization energy:_________________________________________ 42. What is the wavelength for electromagnetic radiation with a frequency of 2.04 x 1011 s-1? 3 x 108m/s =c ...
... b. Largest to smallest electronegativity:________________________________________ c. Largest to smallest ionization energy:_________________________________________ 42. What is the wavelength for electromagnetic radiation with a frequency of 2.04 x 1011 s-1? 3 x 108m/s =c ...
Mongar Higher Secondary School
... The amount of substance containing particles equal to Avogadro’s number. iii) According to electronic concept, a process in which one or more electrons are lost. iv) The fundamental property used for classification of elements in the modern periodic table. v) A salt which is used in some kind of ‘ir ...
... The amount of substance containing particles equal to Avogadro’s number. iii) According to electronic concept, a process in which one or more electrons are lost. iv) The fundamental property used for classification of elements in the modern periodic table. v) A salt which is used in some kind of ‘ir ...
Chapter 3 Discovering the atom and subatomic particles (History of
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
Chapter 3 Discovering the atom and subatomic particles (History of
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
Chapter 2 - OrgSites.com
... Organic chemistry is the study of carbon compounds 1. Most organic compounds contain ___ and ___. 2. Summarize what Stanley Miller was able to demonstrate in 1953. ...
... Organic chemistry is the study of carbon compounds 1. Most organic compounds contain ___ and ___. 2. Summarize what Stanley Miller was able to demonstrate in 1953. ...
Description: This is an advanced placement course designed to
... With the introduction in 1999 of a required laboratory-based question on the free-response section of the AP Chemistry Exam, the inclusion of appropriate experiments into each AP Chemistry course is increasingly important….. It is unlikely that every student will complete all of the 22 laboratory ex ...
... With the introduction in 1999 of a required laboratory-based question on the free-response section of the AP Chemistry Exam, the inclusion of appropriate experiments into each AP Chemistry course is increasingly important….. It is unlikely that every student will complete all of the 22 laboratory ex ...
Chapter 4 Power Point Quiz
... The rapid decomposition of sodium azide, NaN3, to its elements is one of the reactions used to inflate airbags: 2 NaN3 (s) 2 Na (s) + 3 N2 (g) ...
... The rapid decomposition of sodium azide, NaN3, to its elements is one of the reactions used to inflate airbags: 2 NaN3 (s) 2 Na (s) + 3 N2 (g) ...
matter and its reactivity. Objects in the universe are composed of
... 3.3a All matter is made up of atoms. Atoms are far too small to see with a light microscope. 3.3c Atoms may join together in well-defined molecules or may be arranged in regular geometric patterns. 3.3d Interactions among atoms and/or molecules result in chemical reactions. 3.3e The atoms of any on ...
... 3.3a All matter is made up of atoms. Atoms are far too small to see with a light microscope. 3.3c Atoms may join together in well-defined molecules or may be arranged in regular geometric patterns. 3.3d Interactions among atoms and/or molecules result in chemical reactions. 3.3e The atoms of any on ...
8B31A38F-1279-3B00-CDA90244BEA11A7B
... • There are 3 forms bonding atoms: • Ionic—complete transfer of 1 or more electrons from one atom to another (one loses, the other gains) • Covalent—some valence electrons shared between atoms • Metallic – holds atoms of a metal together ...
... • There are 3 forms bonding atoms: • Ionic—complete transfer of 1 or more electrons from one atom to another (one loses, the other gains) • Covalent—some valence electrons shared between atoms • Metallic – holds atoms of a metal together ...
snc 2do unit: chemistry unit test review questions
... 6. Consider a solution with a pH of 3 and a solution with a pH of 5. Which is more acidic? How much more acidic is it? 7. What does the Law of Conservation of Mass state? 8. Describe three tests you can perform to check if an unknown substance is an acid or a base? 9. What type of substances antacid ...
... 6. Consider a solution with a pH of 3 and a solution with a pH of 5. Which is more acidic? How much more acidic is it? 7. What does the Law of Conservation of Mass state? 8. Describe three tests you can perform to check if an unknown substance is an acid or a base? 9. What type of substances antacid ...
Matter and Atoms
... masses B. Nature contains a variety of isotopes C. Isotopes used to find atomic mass of element ...
... masses B. Nature contains a variety of isotopes C. Isotopes used to find atomic mass of element ...
AP Chemistry Syllabus - Tuloso
... illuminate the principles. The following areas should be covered: A. Chemical reactivity and products of chemical reactions B. Relationships in the periodic table: horizontal, vertical, and diagonal with examples from alkali metals, alkaline earth metals, halogens, and the first series of transition ...
... illuminate the principles. The following areas should be covered: A. Chemical reactivity and products of chemical reactions B. Relationships in the periodic table: horizontal, vertical, and diagonal with examples from alkali metals, alkaline earth metals, halogens, and the first series of transition ...
1st Olympiad of Metropolises Chemistry Theoretical Problems
... Furan derivatives can be efficiently converted into other heterocycles. Thus, in 1930th professor of Moscow State University Yu. K. Yuriev developed industrial transformation of furans into pyrroles under heating of furan with ammonia (amines) above 400 C in the presence of alumina. In a laboratory ...
... Furan derivatives can be efficiently converted into other heterocycles. Thus, in 1930th professor of Moscow State University Yu. K. Yuriev developed industrial transformation of furans into pyrroles under heating of furan with ammonia (amines) above 400 C in the presence of alumina. In a laboratory ...
AP CHEMISTRY
... 2. Which of the following pairs of compounds have the same empirical formula? a. Acetylene, C2H2, and benzene, C6H6 b. Ethane, C2H6, and benzene, C6H6 c. Nitrogen dioxide, NO2 , and dinitrogen tetroxide, N2O4 3. D. Diphenyl ether, C12H8O, and phenol, C6H5OHIn an experiment, a 2.514-g sample of calci ...
... 2. Which of the following pairs of compounds have the same empirical formula? a. Acetylene, C2H2, and benzene, C6H6 b. Ethane, C2H6, and benzene, C6H6 c. Nitrogen dioxide, NO2 , and dinitrogen tetroxide, N2O4 3. D. Diphenyl ether, C12H8O, and phenol, C6H5OHIn an experiment, a 2.514-g sample of calci ...
DESCRIPTION FOR THE GENERAL PUBLIC Luminescent materials
... by strong UV light which makes problem in the photostability of the materials under prolonged excitation. Thus, the another desired property (d) is the visible emission induced by safer and cheaper sources of light operating in the NIR range, which is achievable in the so called up-conversion lumine ...
... by strong UV light which makes problem in the photostability of the materials under prolonged excitation. Thus, the another desired property (d) is the visible emission induced by safer and cheaper sources of light operating in the NIR range, which is achievable in the so called up-conversion lumine ...
Trends in the periodic table - Brigham Young University
... Hydrides and hydroxides • Metal hydrides react with water to form metal hydroxides. Because of this, they are __________. The general form is MHx (s) + xH2O (l) → M(OH)x (s) + xH2 (g) ...
... Hydrides and hydroxides • Metal hydrides react with water to form metal hydroxides. Because of this, they are __________. The general form is MHx (s) + xH2O (l) → M(OH)x (s) + xH2 (g) ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.