Chemistry Midterm Review Study Guide 2012
... Non-metal: Brittle, lack luster, poor conductors of heat and electricity ex. O2, Cl2 Metalloids: Semi conductor’s, solid, have characteristics between metals/ nonmetal, B,Si,Ge,As,Sb,Te 8. What do elements in the same group have in common? The same period? Group or family- vertical, have similar che ...
... Non-metal: Brittle, lack luster, poor conductors of heat and electricity ex. O2, Cl2 Metalloids: Semi conductor’s, solid, have characteristics between metals/ nonmetal, B,Si,Ge,As,Sb,Te 8. What do elements in the same group have in common? The same period? Group or family- vertical, have similar che ...
DEPARTMENT OF CHEMISTRY Course Book for M.Sc. in Chemistry
... Mangnese (IV) oxide, Silver carbonate, Oppenauer oxidation, Peroxide, peroxyacids, potassium permanganate, Osmium tetraoxide, Prevost oxidation and Woodward modifications, Periodic acid, Lead Tetraacetate, NBS, DDQ, Chloranil, SeO2. Reduction : Introduction, Catalytic hydrogenation, Homogeneous and ...
... Mangnese (IV) oxide, Silver carbonate, Oppenauer oxidation, Peroxide, peroxyacids, potassium permanganate, Osmium tetraoxide, Prevost oxidation and Woodward modifications, Periodic acid, Lead Tetraacetate, NBS, DDQ, Chloranil, SeO2. Reduction : Introduction, Catalytic hydrogenation, Homogeneous and ...
Regents_Chem_Core_for_review
... IV.8 Atoms attain a stable valence electron configuration by bonding with other atoms. Noble gases have stable valence configurations and tend not to bond. (5.2b) IV.9 Physical properties of substances can be explained in terms of chemical bonds and intermolecular forces. These properties include co ...
... IV.8 Atoms attain a stable valence electron configuration by bonding with other atoms. Noble gases have stable valence configurations and tend not to bond. (5.2b) IV.9 Physical properties of substances can be explained in terms of chemical bonds and intermolecular forces. These properties include co ...
cell molecules
... • A compound is a substance consisting of two or more elements in a fixed ratio. • Table salt (sodium chloride or NaCl) is a compound with equal numbers of chlorine and sodium atoms. • While pure sodium is a metal and chlorine is a gas, their combination forms an edible compound, an emergent proper ...
... • A compound is a substance consisting of two or more elements in a fixed ratio. • Table salt (sodium chloride or NaCl) is a compound with equal numbers of chlorine and sodium atoms. • While pure sodium is a metal and chlorine is a gas, their combination forms an edible compound, an emergent proper ...
Chapter 4 The Structure of Matter
... • 2. often formed between nonmetal atoms • a. can be solids, liquids, or gases • b. usually low melting points except for compounds that form network structures like SiO2 ...
... • 2. often formed between nonmetal atoms • a. can be solids, liquids, or gases • b. usually low melting points except for compounds that form network structures like SiO2 ...
AP Chemistry Summer Assignment
... e. Chromatography from crystallization 31. An extensive property is one that depends on the amount of the sample. Which of the following properties are extensive? a. volume b. density c. temperature d. energy e. melting point. F. pressure 32. A hydrated compound has an analysis of 18.29% Ca, 32.37% ...
... e. Chromatography from crystallization 31. An extensive property is one that depends on the amount of the sample. Which of the following properties are extensive? a. volume b. density c. temperature d. energy e. melting point. F. pressure 32. A hydrated compound has an analysis of 18.29% Ca, 32.37% ...
Tutorial 1
... 1. Use the second member of each group from Group 1A to Group 7A to show that the number of valance electrons on an atom of the element is the same as its group number. 2. Use Lewis dot symbol to show the formation of aluminum oxide (Al 2O3) 3. Explain what an ionic bond is? And name five metals and ...
... 1. Use the second member of each group from Group 1A to Group 7A to show that the number of valance electrons on an atom of the element is the same as its group number. 2. Use Lewis dot symbol to show the formation of aluminum oxide (Al 2O3) 3. Explain what an ionic bond is? And name five metals and ...
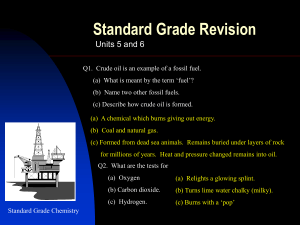
Unit 5 and 6 revsion - Deans Community High School
... Q14. The burning of fossil fuels is a major source of air pollution. (a) Name the acidic gas formed when air in a car engine is sparked. (b) Name the acidic gas formed when coal with a high sulphur content is burned. (c) Name the toxic gas formed when methane is burned in a limited supply of air. (d ...
... Q14. The burning of fossil fuels is a major source of air pollution. (a) Name the acidic gas formed when air in a car engine is sparked. (b) Name the acidic gas formed when coal with a high sulphur content is burned. (c) Name the toxic gas formed when methane is burned in a limited supply of air. (d ...
Course Syllabus - Honors Chemistry
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. a. Atomic number and atomic mass. b. Identify metals, semimetals, nonmetals, halogens, alkali metals, alkaline earth ...
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. a. Atomic number and atomic mass. b. Identify metals, semimetals, nonmetals, halogens, alkali metals, alkaline earth ...
Chapter 7. Statistical Mechanics page 491
... and that I believe this discipline offers a very powerful background for understanding essentially all other areas of chemistry. Even though people like me do not perform laboratory experiments, it is essential that we understand how experiments are done and what elements of molecular behavior they ...
... and that I believe this discipline offers a very powerful background for understanding essentially all other areas of chemistry. Even though people like me do not perform laboratory experiments, it is essential that we understand how experiments are done and what elements of molecular behavior they ...
No Slide Title
... • Ag+ accepts electrons for Cu and is reduced to Ag and Cu loses electrons to Ag+ and is oxidized to Cu+2 in the following redox rxn: 2Ag+(aq) + Cu(s) 2Ag(s) + Cu+2(aq) ...
... • Ag+ accepts electrons for Cu and is reduced to Ag and Cu loses electrons to Ag+ and is oxidized to Cu+2 in the following redox rxn: 2Ag+(aq) + Cu(s) 2Ag(s) + Cu+2(aq) ...
CHAPTER 2
... greater number of oxygen atoms have an “–ate” ending, and the smaller number of oxygen atoms have an “-ite” ending NO3NO2SO42SO32• If more than two exist, the one with the largest number of oxygen atoms have a prefix “per-” and an “-ate” ending, and the smallest number of oxygen atoms have a prefix ...
... greater number of oxygen atoms have an “–ate” ending, and the smaller number of oxygen atoms have an “-ite” ending NO3NO2SO42SO32• If more than two exist, the one with the largest number of oxygen atoms have a prefix “per-” and an “-ate” ending, and the smallest number of oxygen atoms have a prefix ...
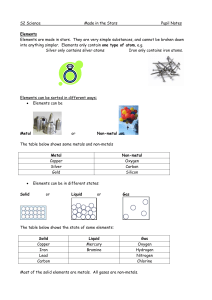
Classification of Matter
... Elements & Symbols • The symbol of an element is often taken from its name. • The first letter is always capitalized. • If an element starts with the same letter as another element, sometime the first two letters are used. • The second letter is always lowercase. • Some elements have symbols that d ...
... Elements & Symbols • The symbol of an element is often taken from its name. • The first letter is always capitalized. • If an element starts with the same letter as another element, sometime the first two letters are used. • The second letter is always lowercase. • Some elements have symbols that d ...
Chapter 4 Notes - Atomic Theory
... Remember: positive charges must = negative charges Ex.1: What is the formula for magnesium phosphide? Magnesium is Mg2+ Phosphorous is P3– Lowest common multiple of 2 and 3 is 6 3 Mg2+ ions & 2 P3– ions (6 +ve’s & 6 –ve’s) ...
... Remember: positive charges must = negative charges Ex.1: What is the formula for magnesium phosphide? Magnesium is Mg2+ Phosphorous is P3– Lowest common multiple of 2 and 3 is 6 3 Mg2+ ions & 2 P3– ions (6 +ve’s & 6 –ve’s) ...
Chapter 2
... The Atomic Theory of Matter Dalton’s Theory of Matter: 1. Each element is composed of extremely small particles call atoms. 2. All atoms of a given element are identical; atoms of different elements are different. 3. Atoms of an element are not changed into different types of atoms by a chemical re ...
... The Atomic Theory of Matter Dalton’s Theory of Matter: 1. Each element is composed of extremely small particles call atoms. 2. All atoms of a given element are identical; atoms of different elements are different. 3. Atoms of an element are not changed into different types of atoms by a chemical re ...
Bonding and Nomenclature
... atoms, or groups of atoms about a central atom in a covalent compound, or charged ion, is determined solely by the repulsions between electron pairs present in the valence shell of the central atom. • The number of electron pairs around the central atom can be determined by writing the Lewis structu ...
... atoms, or groups of atoms about a central atom in a covalent compound, or charged ion, is determined solely by the repulsions between electron pairs present in the valence shell of the central atom. • The number of electron pairs around the central atom can be determined by writing the Lewis structu ...
Chemical Equations
... Most chemical equations give the physical states of the reactants and products: ...
... Most chemical equations give the physical states of the reactants and products: ...
CHEMISTRY 1 FINAL EXAM REVIEW
... 3.) List each as being a physical change or a chemical change: butter melting, butter burning, sugar dissolving in water, a sandwich getting digested. 4.) List each as being a physical or a chemical property: copper sulfate is blue, iron is a solid, water o ...
... 3.) List each as being a physical change or a chemical change: butter melting, butter burning, sugar dissolving in water, a sandwich getting digested. 4.) List each as being a physical or a chemical property: copper sulfate is blue, iron is a solid, water o ...
Oxidation Number Rules
... Examples: N2, Cl2, C, Sn, S8 2. The oxidation number of a simple, monatomic ion is the same as the charge on the ion. Examples: Na+ is +1, Cu+2 is +2, Cu+ is +1, F¯ is -1. 3. The oxidation numbers of some common atoms are: a. Fluorine, the most electronegative element, is -1 in all fluorine containi ...
... Examples: N2, Cl2, C, Sn, S8 2. The oxidation number of a simple, monatomic ion is the same as the charge on the ion. Examples: Na+ is +1, Cu+2 is +2, Cu+ is +1, F¯ is -1. 3. The oxidation numbers of some common atoms are: a. Fluorine, the most electronegative element, is -1 in all fluorine containi ...
Chapter 2: Chemistry Level
... High heat capacity – absorbs and releases large amounts of heat before changing temperature High heat of vaporization – changing from a liquid to a gas requires large amounts of heat ...
... High heat capacity – absorbs and releases large amounts of heat before changing temperature High heat of vaporization – changing from a liquid to a gas requires large amounts of heat ...
Unit 3.2 worksheet 4 atomic model of matter
... Bonding. Why and how do atoms combine to form compounds? In this unit, we will draw Lewis structures to describe bonding and. The Periodic Table by WebElements. The periodic table is an arrangment of the chemical elements ordered by atomic number so that periodic properties of the elements. Getting ...
... Bonding. Why and how do atoms combine to form compounds? In this unit, we will draw Lewis structures to describe bonding and. The Periodic Table by WebElements. The periodic table is an arrangment of the chemical elements ordered by atomic number so that periodic properties of the elements. Getting ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.