Chapter 1 Chemistry: Matter and Measurement
... Chapter 1 - Chemistry: Matter and Measurements Introduction: Chemistry is concerned with matter and energy and how the two interact with each other. ...
... Chapter 1 - Chemistry: Matter and Measurements Introduction: Chemistry is concerned with matter and energy and how the two interact with each other. ...
CHEM 250Q
... A sealed test tube containing 8 grams of iron filings and 5 grams of sulfur is heated until the material in the test tube glows bright red. Which is true about the reaction? A. ...
... A sealed test tube containing 8 grams of iron filings and 5 grams of sulfur is heated until the material in the test tube glows bright red. Which is true about the reaction? A. ...
Review Material
... Since the molecules of a gas are far apart, the forces of attraction between them are negligible. The molecules of a gas are in continual, random, and rapid motion. The average kinetic energy of gas molecules depends only on the gas temperature, and can be expressed by EK T. Gas molecules collide ...
... Since the molecules of a gas are far apart, the forces of attraction between them are negligible. The molecules of a gas are in continual, random, and rapid motion. The average kinetic energy of gas molecules depends only on the gas temperature, and can be expressed by EK T. Gas molecules collide ...
Chemistry 20H
... Physical reactions also include subdivision, when a substance is broken into pieces. If a rock is ground into powder, the powder is the same substance as the rock was; the subdivision has resulted only in a physical change. A chemical change results when the atoms of one or more substances are rearr ...
... Physical reactions also include subdivision, when a substance is broken into pieces. If a rock is ground into powder, the powder is the same substance as the rock was; the subdivision has resulted only in a physical change. A chemical change results when the atoms of one or more substances are rearr ...
Chapter 2_Application Problems
... • Copper atoms can combine with zinc atoms to make gold atoms – incorrect; according to Dalton, atoms of one element cannot turn into atoms of another element by a chemical reaction. He knew this because if atoms could change it would change the total mass and violate the Law of Conservation of Mass ...
... • Copper atoms can combine with zinc atoms to make gold atoms – incorrect; according to Dalton, atoms of one element cannot turn into atoms of another element by a chemical reaction. He knew this because if atoms could change it would change the total mass and violate the Law of Conservation of Mass ...
Chapter 12
... Use the following rules to properly name hydrocarbon molecules: –1. Identify the longest, continuous chain of C atoms and name it. –2. # the C atoms in the chain from the end nearest an alkyl group. –3. # and name the attached alkyl group(s). –4. If more than one alkyl group: •first substituent must ...
... Use the following rules to properly name hydrocarbon molecules: –1. Identify the longest, continuous chain of C atoms and name it. –2. # the C atoms in the chain from the end nearest an alkyl group. –3. # and name the attached alkyl group(s). –4. If more than one alkyl group: •first substituent must ...
2 - FacultyWeb
... the smallest unit of matter that still retains the properties of an element. composed of even smaller parts, called subatomic particles. Neutrons and protons: packed together to form a dense core, the Atomic Nucleus, at the center of an atom. ...
... the smallest unit of matter that still retains the properties of an element. composed of even smaller parts, called subatomic particles. Neutrons and protons: packed together to form a dense core, the Atomic Nucleus, at the center of an atom. ...
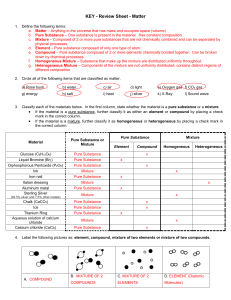
Matter Test Review Sheet
... c. Mixture – Composed of 2 or more pure substances that are not chemically combined and can be separated by physical processes. d. Element – Pure substance composed of only one type of atom. e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by ...
... c. Mixture – Composed of 2 or more pure substances that are not chemically combined and can be separated by physical processes. d. Element – Pure substance composed of only one type of atom. e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by ...
James Moir as Inorganic Chemist
... Scotland, where he graduated from the University of Aberdeen with a DSc in 1902. He first acted as a chemist in the laboratory of a gold mining company and in 1904 was appointed chemist to the Transvaal Department of Mines. In 1914 he joined the staff of the Government Analyst at the Government Chem ...
... Scotland, where he graduated from the University of Aberdeen with a DSc in 1902. He first acted as a chemist in the laboratory of a gold mining company and in 1904 was appointed chemist to the Transvaal Department of Mines. In 1914 he joined the staff of the Government Analyst at the Government Chem ...
Standards Practice
... 19. If 1 mol of gas has a volume of 22.4 L at standard temperature and pressure (STP), how much volume would 0.5 mol of the same gas have? A. 0.5 L B. 11.2 L C. 22.4 L D. 44.8 L ...
... 19. If 1 mol of gas has a volume of 22.4 L at standard temperature and pressure (STP), how much volume would 0.5 mol of the same gas have? A. 0.5 L B. 11.2 L C. 22.4 L D. 44.8 L ...
Name_____________________________________ Chemistry
... An example of a chemical change is a. sanding wood. c. milk going sour. b. melting ice. d. vaporizing gasoline. ____ 12. A physical change occurs when a a. peach spoils. c. bracelet turns your wrist green. b. copper bowl tarnishes. d. glue gun melts a glue stick. ____ 13. The state of matter in whic ...
... An example of a chemical change is a. sanding wood. c. milk going sour. b. melting ice. d. vaporizing gasoline. ____ 12. A physical change occurs when a a. peach spoils. c. bracelet turns your wrist green. b. copper bowl tarnishes. d. glue gun melts a glue stick. ____ 13. The state of matter in whic ...
Matter and Change
... • What is the difference between an element and a pure substance? • Provide an example of an element and a compound. • Where would you find a list of elements? ...
... • What is the difference between an element and a pure substance? • Provide an example of an element and a compound. • Where would you find a list of elements? ...
Ionic Bonding - petersonORHS
... they are NOT part of the formula, they just help us get the correct subscripts!) Al2O3 ...
... they are NOT part of the formula, they just help us get the correct subscripts!) Al2O3 ...
Chemistry- CST Review
... Standard 4 – Gases and their Properties 1. What causes gas pressure in terms of kinetic theory? Gas pressure is caused by the random motion of the gas molecules. 2. If someone sprays perfume at the front of the room, will the people in the back of the room eventually be able to smell it? Why? Expla ...
... Standard 4 – Gases and their Properties 1. What causes gas pressure in terms of kinetic theory? Gas pressure is caused by the random motion of the gas molecules. 2. If someone sprays perfume at the front of the room, will the people in the back of the room eventually be able to smell it? Why? Expla ...
Chapter 8 - Chemical Equations
... * because there is oxygen in every compound in the equation, it may be helpful to count the number of a polyatomic ion, rather than splitting the polyatomic ion into its elements and then counting.* EXAMPLE #6: ...
... * because there is oxygen in every compound in the equation, it may be helpful to count the number of a polyatomic ion, rather than splitting the polyatomic ion into its elements and then counting.* EXAMPLE #6: ...
Chem EOC Review Cumulative Free Response
... 72) Atoms form a mostly covalent bond if they have a _________ difference in electronegativity. 73) A _______________ compound is one that contains covalent bonds. 74) List the 7 diatomic molecules. 75) What type of bond exists in an N2 molecule? ______________ ______________ 76) Name a diatomic tha ...
... 72) Atoms form a mostly covalent bond if they have a _________ difference in electronegativity. 73) A _______________ compound is one that contains covalent bonds. 74) List the 7 diatomic molecules. 75) What type of bond exists in an N2 molecule? ______________ ______________ 76) Name a diatomic tha ...
one
... – Once one element is balanced, proceed to balance another, and another, until all elements are balanced. – Balance chemical formulas by placing coefficients in front of them. Do not add subscripts, because this will change the formulas. – Coefficients are multipliers, so if we write 2 H2O it denote ...
... – Once one element is balanced, proceed to balance another, and another, until all elements are balanced. – Balance chemical formulas by placing coefficients in front of them. Do not add subscripts, because this will change the formulas. – Coefficients are multipliers, so if we write 2 H2O it denote ...
UNIT 2 – Chemical Quantities
... Ex 3. During WWI, chlorine gas was used as a weapon. When chlorine gas enters the lungs it reacts with water and forms corrosive hydrochloric acid and oxygen gas. How many molecules of chlorine react to produce 5.0g of HCl? ...
... Ex 3. During WWI, chlorine gas was used as a weapon. When chlorine gas enters the lungs it reacts with water and forms corrosive hydrochloric acid and oxygen gas. How many molecules of chlorine react to produce 5.0g of HCl? ...
1 FORMATION OF THE ATOMIC THEORY
... 1.1 The birth of chemistry Modern chemistry was initiated by the French chemist Antoine Laurent Lavoisier (1743-1794). He established the law of conservation of mass for chemical reactions, and revealed the role of oxygen in combustion. Based on these principles, chemistry progressed in the right di ...
... 1.1 The birth of chemistry Modern chemistry was initiated by the French chemist Antoine Laurent Lavoisier (1743-1794). He established the law of conservation of mass for chemical reactions, and revealed the role of oxygen in combustion. Based on these principles, chemistry progressed in the right di ...
Atomic number - River Dell Regional School District
... Wrote New System of Chemical Philosophy in ...
... Wrote New System of Chemical Philosophy in ...
C6-Chemical Reactions
... Do these 4 indications of chemical change ALWAYS mean chemical changes?? Transfer of energy Fire always means chemical change Heat, cooling, and light can indicate either type of change. To be ...
... Do these 4 indications of chemical change ALWAYS mean chemical changes?? Transfer of energy Fire always means chemical change Heat, cooling, and light can indicate either type of change. To be ...
FREE Sample Here
... 11) Ionic bonds are formed when A) atoms share electrons. B) two or more atoms lose electrons at the same time. C) electrons are completely transferred from one atom to another. D) hydrogen forms bonds with negatively charged atoms in the same or different molecule. E) a pair of electrons is shared ...
... 11) Ionic bonds are formed when A) atoms share electrons. B) two or more atoms lose electrons at the same time. C) electrons are completely transferred from one atom to another. D) hydrogen forms bonds with negatively charged atoms in the same or different molecule. E) a pair of electrons is shared ...