Review for SNC 2P Chemistry Unit(SPRING 2014)
... (f) A reaction that involves the combination of two elements._______________________ ...
... (f) A reaction that involves the combination of two elements._______________________ ...
Chapter 10_Handouts_6
... The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the elements according to atomic nu ...
... The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the elements according to atomic nu ...
Chapter 10 Handouts_1
... •Active elements liberate more heat when they react than do inactive elements. •Active elements usually form stable compounds. ...
... •Active elements liberate more heat when they react than do inactive elements. •Active elements usually form stable compounds. ...
Chapter 10 Handouts - Bakersfield College
... •Active elements liberate more heat when they react than do inactive elements. •Active elements usually form stable compounds. ...
... •Active elements liberate more heat when they react than do inactive elements. •Active elements usually form stable compounds. ...
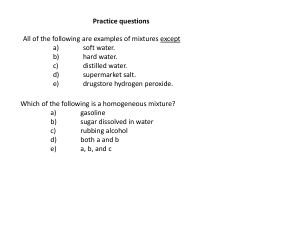
Practice questions
... Maleic acid contains 41.4% carbon, 3.47% hydrogen, and 55.1% oxygen by mass. A 0.050-mol sample of this compound weighs 5.80 g. What is the molecular formula of ...
... Maleic acid contains 41.4% carbon, 3.47% hydrogen, and 55.1% oxygen by mass. A 0.050-mol sample of this compound weighs 5.80 g. What is the molecular formula of ...
chemistry i - surrattchemistry
... 3. A biochemist is performing an experiment to determine the effects of Chemical X on the growth of bacteria. Which tube is the experimental control? a. Test tube 1 b. Test tube 2 c. Test tube 3 d. Test tube 4 Objective 2.01: Analyze the historical development of the current atomic theory. 4. Which ...
... 3. A biochemist is performing an experiment to determine the effects of Chemical X on the growth of bacteria. Which tube is the experimental control? a. Test tube 1 b. Test tube 2 c. Test tube 3 d. Test tube 4 Objective 2.01: Analyze the historical development of the current atomic theory. 4. Which ...
Writing Formulas
... Remember the algebraic sum of the ions' oxidation numbers must equal zero. (Balance) Learn the polyatomic ions. Learn those ions with multiple oxidation numbers and use Roman numerals to indicate the charge. ...
... Remember the algebraic sum of the ions' oxidation numbers must equal zero. (Balance) Learn the polyatomic ions. Learn those ions with multiple oxidation numbers and use Roman numerals to indicate the charge. ...
Balancing Chemical Equations Lab
... 1. Using your set of cards, replicate the chemical equation onto your desk. Record the following results into Table 1: 2. Identify the elements on the reactant side. 3. Count the number of atoms for each element. 4. Identify the elements on the product side. 5. Count the number of atoms on the produ ...
... 1. Using your set of cards, replicate the chemical equation onto your desk. Record the following results into Table 1: 2. Identify the elements on the reactant side. 3. Count the number of atoms for each element. 4. Identify the elements on the product side. 5. Count the number of atoms on the produ ...
Beryllium isotopes in geochronology Cosmogenic Be and Be
... fission – the spontaneous (or induced by particle collision) splitting of a heavy nucleus into a pair (only rarely more) of nearly equal fission fragments (fission products) generally with some neutrons. Fission is accompanied by the release of a large quantity of energy. [return] gamma rays (gamma ...
... fission – the spontaneous (or induced by particle collision) splitting of a heavy nucleus into a pair (only rarely more) of nearly equal fission fragments (fission products) generally with some neutrons. Fission is accompanied by the release of a large quantity of energy. [return] gamma rays (gamma ...
Metals, Nonmetals, and Metalloids (Vocabulary)
... are arranged by properties and are represented by one or two letter chemical symbols. ...
... are arranged by properties and are represented by one or two letter chemical symbols. ...
Metals, Nonmetals, and Metalloids (Vocabulary)
... Metals, Nonmetals, and Metalloids (Vocabulary) ...
... Metals, Nonmetals, and Metalloids (Vocabulary) ...
How are Molecules Depicted? - Belle Vernon Area School District
... E level of an atom and determines the chemical properties Lewis Structure = a structure in which e- are represented by dots: ...
... E level of an atom and determines the chemical properties Lewis Structure = a structure in which e- are represented by dots: ...
1st Semester Exam in High School Chemistry
... refrigerant and also as a cleaning agent. Prior to the 1950s, carbon tetrachloride was manufactured by the chlorination of carbon disulfide: CS2 + 3 Cl2 → CCl4 + S2Cl2 but now it is mainly produced from methane: CH4 + 4 Cl2 → CCl4 + 4 HCl How many grams of carbon tetrachloride can be produced from r ...
... refrigerant and also as a cleaning agent. Prior to the 1950s, carbon tetrachloride was manufactured by the chlorination of carbon disulfide: CS2 + 3 Cl2 → CCl4 + S2Cl2 but now it is mainly produced from methane: CH4 + 4 Cl2 → CCl4 + 4 HCl How many grams of carbon tetrachloride can be produced from r ...
Chemistry FINAL: CONTENT Review Packet
... _______________________is made from two or more substances that are physically combined The ability to do work is known as ________________ ________________________ are substances that are made up of only one type of atom ____________________________ is anything that has both mass and volume _______ ...
... _______________________is made from two or more substances that are physically combined The ability to do work is known as ________________ ________________________ are substances that are made up of only one type of atom ____________________________ is anything that has both mass and volume _______ ...
Glossary (PDF file)
... growth of plants. The only factor you would change in the experiment would be the amount of water the plants receive. All other factors, such as the amount of sunlight the plants receive, the original size of the plants, and the type of soil the plants are grown in, must stay the same, or constant. ...
... growth of plants. The only factor you would change in the experiment would be the amount of water the plants receive. All other factors, such as the amount of sunlight the plants receive, the original size of the plants, and the type of soil the plants are grown in, must stay the same, or constant. ...
MYP 10 PeriodicityWS
... 5(a) Draw a diagram to show the structure of sodium chloride. Explain, in terms of bonding, why sodium chloride has a high melting point. (b) Lithium reacts with water. Write an equation for the reaction and state two observations that could be made during the reaction. [SL paper 2, Nov 05] 6 (a) Fo ...
... 5(a) Draw a diagram to show the structure of sodium chloride. Explain, in terms of bonding, why sodium chloride has a high melting point. (b) Lithium reacts with water. Write an equation for the reaction and state two observations that could be made during the reaction. [SL paper 2, Nov 05] 6 (a) Fo ...
7R CHEMISTRY 1 REVIEW
... 2. If an element is divided into smaller and smaller parts, the smallest particle obtained would be a (an) A) molecule. B) compound. C) mixture. D) atom. 3. The fact that iron cannot be changed into a simpler form indicates that iron is a (an) A) compound. B) molecule. C) element. ...
... 2. If an element is divided into smaller and smaller parts, the smallest particle obtained would be a (an) A) molecule. B) compound. C) mixture. D) atom. 3. The fact that iron cannot be changed into a simpler form indicates that iron is a (an) A) compound. B) molecule. C) element. ...
Semester Exam Review Guide
... a. valence electrons are increasing b. the total number of protons, electrons, and neutrons is increasing c. electrons are repelling from each other in the valence shell d. elements are becoming very reactive 17. The atomic mass number is equal to the number of a. protons b. neutrons c. protons and ...
... a. valence electrons are increasing b. the total number of protons, electrons, and neutrons is increasing c. electrons are repelling from each other in the valence shell d. elements are becoming very reactive 17. The atomic mass number is equal to the number of a. protons b. neutrons c. protons and ...
document
... G. A reaction in which two reactant compounds switch ions. 9. Decomposition Reaction A H. This number tells the number of atoms of one element in a 10. Single Displacement Reaction O compound. I. Bonds formed by gaining and losing 11. Double Displacement Reaction G electrons. J. A group of atoms tha ...
... G. A reaction in which two reactant compounds switch ions. 9. Decomposition Reaction A H. This number tells the number of atoms of one element in a 10. Single Displacement Reaction O compound. I. Bonds formed by gaining and losing 11. Double Displacement Reaction G electrons. J. A group of atoms tha ...
Chem 152 Chapter 4
... – hydrogen and oxygen react to form water. – 2H2 + O2 2H2O – Reactants on left; products on right. – Heat represented by . Conservation of Mass – No change is observed in the total mass of the substances involved in a chemical ...
... – hydrogen and oxygen react to form water. – 2H2 + O2 2H2O – Reactants on left; products on right. – Heat represented by . Conservation of Mass – No change is observed in the total mass of the substances involved in a chemical ...
Matter in Chemistry
... Boiling the egg: when you use high heat to boil an egg, it causes a chemical reaction between the yolk and the white that leaves a green film around the yolk. That film is iron sulfide, caused by iron in the yolk reacting with hydrogen sulfide in the white (it won't hurt you to eat it, and the egg w ...
... Boiling the egg: when you use high heat to boil an egg, it causes a chemical reaction between the yolk and the white that leaves a green film around the yolk. That film is iron sulfide, caused by iron in the yolk reacting with hydrogen sulfide in the white (it won't hurt you to eat it, and the egg w ...
Name________________________ Midterm Review Date
... nucleus has a negative charge. B) An atom has hardly any empty space, and the nucleus has a negative charge. C) An atom is mainly empty space, and the nucleus has a positive charge. D) An atom has hardly any empty space, and the nucleus has a positive charge. 18. Which element is an alkali metal? A) ...
... nucleus has a negative charge. B) An atom has hardly any empty space, and the nucleus has a negative charge. C) An atom is mainly empty space, and the nucleus has a positive charge. D) An atom has hardly any empty space, and the nucleus has a positive charge. 18. Which element is an alkali metal? A) ...
Chemistry FINAL: CONTENT Review Packet
... 4. Define pH and draw the pH scale showing the locations of acids, bases, and neutral substances. ...
... 4. Define pH and draw the pH scale showing the locations of acids, bases, and neutral substances. ...