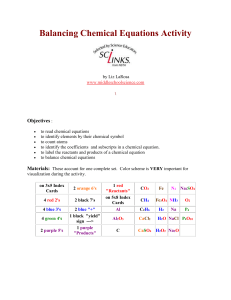
Balancing Chemical Equations Activity by Liz LaRosa www
... Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier and quicker to locate the elements that you are trying to balance. If everything is in black ink, its harder to distinguish the equation contents. I ...
... Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier and quicker to locate the elements that you are trying to balance. If everything is in black ink, its harder to distinguish the equation contents. I ...
Asbtracts of Talks at ICEC 2014 - Association of Chemistry Teachers
... Indian Institute of Science, Bangalore, India Challenges in Organic Chemistry Education at UG and PG levels As a sub-discipline, Organic chemistry is liked by a majority of undergraduate Chemistry students, and more than 50% of Masters’ students in Chemistry eventually specialize in Organic Chemistr ...
... Indian Institute of Science, Bangalore, India Challenges in Organic Chemistry Education at UG and PG levels As a sub-discipline, Organic chemistry is liked by a majority of undergraduate Chemistry students, and more than 50% of Masters’ students in Chemistry eventually specialize in Organic Chemistr ...
CHAPTER 2: ATOMS, MOLECULES AND IONS ULES AND IONS
... Example: 5 liters of oxygen and 5 liters of nitrogen contain same number of particles! - But it is not true (because different atoms have different size and properties). To understand about the atoms, we need to know about what atoms made of, and how do the various atoms differ? ...
... Example: 5 liters of oxygen and 5 liters of nitrogen contain same number of particles! - But it is not true (because different atoms have different size and properties). To understand about the atoms, we need to know about what atoms made of, and how do the various atoms differ? ...
Chemical Reactions
... • Skeletal equations - show identities of reactants and products H2 + O2 → H2O ...
... • Skeletal equations - show identities of reactants and products H2 + O2 → H2O ...
Chem 101 Readiness Guide_2-09
... Students are encouraged to arrive at least a half-hour prior to any scheduled exam. Date of Exam: Plan on arriving at least a half-hour early, to the exam of choice. Main Campus exams are held in Building F5, Room 106. South Gate Educational Center exams are held in Room 120. You must remember to br ...
... Students are encouraged to arrive at least a half-hour prior to any scheduled exam. Date of Exam: Plan on arriving at least a half-hour early, to the exam of choice. Main Campus exams are held in Building F5, Room 106. South Gate Educational Center exams are held in Room 120. You must remember to br ...
AP Chemistry Summer Packet ANSWERS
... 23. Classify each of the following as a mixture or a pure substance. Of the pure substances, which are elements and which are compounds? a. Water – PS (compound) f. Uranium – PS (element) b. Blood - M g. Wine – could be either c. The oceans - M h. Leather – could be either d. Iron – PS (element) i. ...
... 23. Classify each of the following as a mixture or a pure substance. Of the pure substances, which are elements and which are compounds? a. Water – PS (compound) f. Uranium – PS (element) b. Blood - M g. Wine – could be either c. The oceans - M h. Leather – could be either d. Iron – PS (element) i. ...
Here are the answers and work for your summer packet.
... 23. Classify each of the following as a mixture or a pure substance. Of the pure substances, which are elements and which are compounds? a. Water – PS (compound) f. Uranium – PS (element) b. Blood - M g. Wine – could be either c. The oceans - M h. Leather – could be either d. Iron – PS (element) i. ...
... 23. Classify each of the following as a mixture or a pure substance. Of the pure substances, which are elements and which are compounds? a. Water – PS (compound) f. Uranium – PS (element) b. Blood - M g. Wine – could be either c. The oceans - M h. Leather – could be either d. Iron – PS (element) i. ...
Chemical Equations
... The reactants that enter into a reaction The products that are formed by the reaction The relative amounts of each substance used and ...
... The reactants that enter into a reaction The products that are formed by the reaction The relative amounts of each substance used and ...
Vocabulary CHEM121
... Compounds may be divided into 2 general types: 1. Molecular (covalent) compounds are combinations of non-metals 2. Ionic (contains ions) includes: Acids: anything giving H+ when dissolved in water Bases: anything giving OH- when dissolved in water Salts: all other ionic materials Formulas of molecul ...
... Compounds may be divided into 2 general types: 1. Molecular (covalent) compounds are combinations of non-metals 2. Ionic (contains ions) includes: Acids: anything giving H+ when dissolved in water Bases: anything giving OH- when dissolved in water Salts: all other ionic materials Formulas of molecul ...
Chemical reactions alter arrangements of atoms.
... substances formed by a chemical reaction. In the burning of natural gas, carbon dioxide (CO2) and water (H2O) are the products formed by the reaction. Reactants and products can be elements or compounds, depending on the reaction taking place. During a chemical reaction, bonds between atoms in the r ...
... substances formed by a chemical reaction. In the burning of natural gas, carbon dioxide (CO2) and water (H2O) are the products formed by the reaction. Reactants and products can be elements or compounds, depending on the reaction taking place. During a chemical reaction, bonds between atoms in the r ...
Atomic Theory - New Senior Secondary Curriculum Goals
... mass and volume of gas, the temperature of the gas sample varies in direct proportion to its pressure. It should be noted that the pressure law (i) is not absolute – the behaviour of real gases can deviate from it; and (ii) does not explain why gases, real or ideal, behave like this anyway. To make ...
... mass and volume of gas, the temperature of the gas sample varies in direct proportion to its pressure. It should be noted that the pressure law (i) is not absolute – the behaviour of real gases can deviate from it; and (ii) does not explain why gases, real or ideal, behave like this anyway. To make ...
Practice problems for chapter 1, 2 and 3 1) A small amount of salt
... Practice problems for chapter 1, 2 and 3 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substa ...
... Practice problems for chapter 1, 2 and 3 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substa ...
3 - Greene County ESC
... properties of matter, the properties of materials and objects, chemical reactions and the conservation of matter. In addition, it includes understanding the nature, transfer and conservation of energy; motion and the forces affecting motion; and the nature of waves and interactions of matter and ene ...
... properties of matter, the properties of materials and objects, chemical reactions and the conservation of matter. In addition, it includes understanding the nature, transfer and conservation of energy; motion and the forces affecting motion; and the nature of waves and interactions of matter and ene ...
Bennett Department of Chemistry - WVU Catalog
... The bachelor of science with a major in chemistry is approved by the American Chemical Society. This program is for students who desire to qualify for professional positions in industrial and governmental laboratories as well as those who plan to do graduate work in chemistry or allied areas in prep ...
... The bachelor of science with a major in chemistry is approved by the American Chemical Society. This program is for students who desire to qualify for professional positions in industrial and governmental laboratories as well as those who plan to do graduate work in chemistry or allied areas in prep ...
The Basics - I`m a faculty member, and I need web space. What
... • The mole ratios can be obtained from the coefficients in the balanced chemical equation. • What are the mole ratios in this problem? • Mole ratios can be used as conversion factors to predict the amount of any reactant or product involved in a reaction if the amount of another reactant and/or prod ...
... • The mole ratios can be obtained from the coefficients in the balanced chemical equation. • What are the mole ratios in this problem? • Mole ratios can be used as conversion factors to predict the amount of any reactant or product involved in a reaction if the amount of another reactant and/or prod ...
9077590 Chem. Rege. Jan. 01
... If you wish to change an answer, erase your first penciled circle and then circle with pencil the number of the answer you want. After you have completed the examination and you have decided that all of the circled answers represent your best judgment, signal a proctor and turn in all examination ma ...
... If you wish to change an answer, erase your first penciled circle and then circle with pencil the number of the answer you want. After you have completed the examination and you have decided that all of the circled answers represent your best judgment, signal a proctor and turn in all examination ma ...
Chapter 11 Chemical Reactions
... (aq) after the formula = dissolved in water, an aqueous solution: NaCl(aq) is a salt water solution used after a product indicates a gas has been produced: H2↑ used after a product indicates a solid has been produced: PbI2↓ ...
... (aq) after the formula = dissolved in water, an aqueous solution: NaCl(aq) is a salt water solution used after a product indicates a gas has been produced: H2↑ used after a product indicates a solid has been produced: PbI2↓ ...
CST REVIEW Percent Error 1. 2. What is the formula for density?
... 108. Boyle’s law says that as volume decreases the pressure ____ (decrease/increase). 109. A gas at 1.0 atm has its volume raised from 2.0L to 8.0 L. What is the new pressure? 110. Charles’ law states that as temperature increases the volume ____ (decrease/increase). 111. If a balloon has a volume o ...
... 108. Boyle’s law says that as volume decreases the pressure ____ (decrease/increase). 109. A gas at 1.0 atm has its volume raised from 2.0L to 8.0 L. What is the new pressure? 110. Charles’ law states that as temperature increases the volume ____ (decrease/increase). 111. If a balloon has a volume o ...
the Language of Chemistry
... Some questions that I have been often asked by the students and parents alike have been—“What do we do by learning Chemistry?” “For a student of Literature or even History what role can Chemistry play?” But the answers came very simply. A mother administering her child a tablet for fever should know ...
... Some questions that I have been often asked by the students and parents alike have been—“What do we do by learning Chemistry?” “For a student of Literature or even History what role can Chemistry play?” But the answers came very simply. A mother administering her child a tablet for fever should know ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.























