CH. 15 Notes
... to the right of the chemical symbols are called Subscripts, and notes the number of atoms of that element. A number in front of a chemical formula is a coefficient and it is multiplied by the subscript of all the atoms that are in the formula ...
... to the right of the chemical symbols are called Subscripts, and notes the number of atoms of that element. A number in front of a chemical formula is a coefficient and it is multiplied by the subscript of all the atoms that are in the formula ...
Radioisotopes
... (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number o ...
... (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number o ...
rp oc4
... What is the empirical and molecular formula of a compound with the following percent composition: P = 26.7%, N = 12.1 %, Cl = 61.2%. The molar mass of the compound is 695 g/mol. ...
... What is the empirical and molecular formula of a compound with the following percent composition: P = 26.7%, N = 12.1 %, Cl = 61.2%. The molar mass of the compound is 695 g/mol. ...
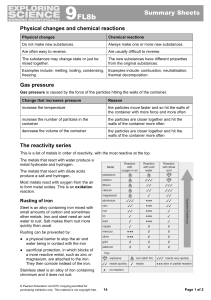
9F Reactivity - Parrs Wood High School
... ● The metals lower in the reactivity series are easier to extract from their ores and they have been available to use as the pure elements for much longer. ● Metals from zinc downwards in the reactivity series can be extracted from their ores by heating with carbon. ● Metals above zinc in the reacti ...
... ● The metals lower in the reactivity series are easier to extract from their ores and they have been available to use as the pure elements for much longer. ● Metals from zinc downwards in the reactivity series can be extracted from their ores by heating with carbon. ● Metals above zinc in the reacti ...
Chapter 2 Notes
... 3. chemical properties = describe how one substance changes when it reacts with other substances; example: iron changes to rust when it reacts to water and oxygen ***may indicate a chemical reaction: a. color change b. gas produced c. temperature change 4. Ions- electrically charged atoms; atom eith ...
... 3. chemical properties = describe how one substance changes when it reacts with other substances; example: iron changes to rust when it reacts to water and oxygen ***may indicate a chemical reaction: a. color change b. gas produced c. temperature change 4. Ions- electrically charged atoms; atom eith ...
Science notes on Atoms, Periodic table
... 1st discovered & named by Democritus, who believed it was a small indivisible particle of matter. Aristotle believed that it was infinitely divisible (you could keep on cutting it forever). He also believed that everything was composed of 5 elements: water, earth, fire, air & aether John Dalton then ...
... 1st discovered & named by Democritus, who believed it was a small indivisible particle of matter. Aristotle believed that it was infinitely divisible (you could keep on cutting it forever). He also believed that everything was composed of 5 elements: water, earth, fire, air & aether John Dalton then ...
Grades 9-12 Chemistry California Content Standards
... b. the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions: change in mass (calculated by E=mc_) is small but significant in nuclear reactions. c. many naturally occurring isotopes of elements are radioactive, as are isotopes formed i ...
... b. the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions: change in mass (calculated by E=mc_) is small but significant in nuclear reactions. c. many naturally occurring isotopes of elements are radioactive, as are isotopes formed i ...
atoms
... proportions by mass of the constituent elements. Consider the compound water made up of two atoms of hydrogen (H) for every atoms of oxygen (O) Can be presented chemical formula H20 Two samples describes below have the same proportions of the two elements Exp: determine the percent by mass of h ...
... proportions by mass of the constituent elements. Consider the compound water made up of two atoms of hydrogen (H) for every atoms of oxygen (O) Can be presented chemical formula H20 Two samples describes below have the same proportions of the two elements Exp: determine the percent by mass of h ...
atoms
... Most of mass and all of positive charge of an atom are centered in a very small region called nucleus. The remainder of the atom is mostly empty space The magnitude of the positive charge is different for the different atoms and is approximately one-half the atomic weight of the element There a ...
... Most of mass and all of positive charge of an atom are centered in a very small region called nucleus. The remainder of the atom is mostly empty space The magnitude of the positive charge is different for the different atoms and is approximately one-half the atomic weight of the element There a ...
*6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing
... Lesson 5.1: Observing Chemical Change *Changes in matter can be described in terms of physical changes and chemical changes. *Chemical reactions involve changes in properties and changes in energy that you can often observe. Physical change-any change that alters the form or appearance of a substanc ...
... Lesson 5.1: Observing Chemical Change *Changes in matter can be described in terms of physical changes and chemical changes. *Chemical reactions involve changes in properties and changes in energy that you can often observe. Physical change-any change that alters the form or appearance of a substanc ...
CHAPTER 2
... electrons orbiting around the nucleus. An atom may loose or gain one or more electrons – the resulting particle is called an ION If the atom loses electron(s), it becomes a cation (positively charged) If the atom gains electron(s), it becomes an anion (positively charged) ...
... electrons orbiting around the nucleus. An atom may loose or gain one or more electrons – the resulting particle is called an ION If the atom loses electron(s), it becomes a cation (positively charged) If the atom gains electron(s), it becomes an anion (positively charged) ...
COUNTING ATOMS
... CHEMICAL EQUATIONS • Some equations have a coefficient. • Coefficients are numbers that appear before elements in a chemical equation that change the number of reactants or products. • Example: • H2 + O2 H20 • The correct way to write this equation is: • 2H2 + O2 2H2O • The coefficients change ...
... CHEMICAL EQUATIONS • Some equations have a coefficient. • Coefficients are numbers that appear before elements in a chemical equation that change the number of reactants or products. • Example: • H2 + O2 H20 • The correct way to write this equation is: • 2H2 + O2 2H2O • The coefficients change ...
Chemistry - School District of Springfield Township
... Unit III: The Organization of Matter • Explain how the relationships of chemical properties of elements are represented in the repeating patterns of the Periodic Table using the periodic law. • Identify and describe the important trends that exist on the Periodic Table and discuss how each trend ref ...
... Unit III: The Organization of Matter • Explain how the relationships of chemical properties of elements are represented in the repeating patterns of the Periodic Table using the periodic law. • Identify and describe the important trends that exist on the Periodic Table and discuss how each trend ref ...
Grade 10 NSC Chemistry Curriculum
... (new chemical substances are formed) • Describe examples of a chemical change that could include - the decomposition of hydrogen peroxide to form water and oxygen; and - the synthesis reaction that occurs when hydrogen burns in oxygen to form water. (Why do we consider these reactions to be chemical ...
... (new chemical substances are formed) • Describe examples of a chemical change that could include - the decomposition of hydrogen peroxide to form water and oxygen; and - the synthesis reaction that occurs when hydrogen burns in oxygen to form water. (Why do we consider these reactions to be chemical ...
Practical, Asymmetric Redox-Neutral Chemical Synthesis via Borrowing Hydrogen
... Synthetic chemistry is considered to be the “central science” as it provides the toolbox for various applied areas such as pharmaceuticals, chemical biology and material science. Due to resource constraints, the current trend in synthetic chemistry is not simply about preparing molecules of specific ...
... Synthetic chemistry is considered to be the “central science” as it provides the toolbox for various applied areas such as pharmaceuticals, chemical biology and material science. Due to resource constraints, the current trend in synthetic chemistry is not simply about preparing molecules of specific ...
29.2 Chemical Bonds
... The concept of temperature and changes of phase between solid, liquid, and gas are traditionally considered part of chemistry, as are the gas laws. These kinds of changes in matter are called physical changes, because matter changes physical form but one substance does not change into a complete ...
... The concept of temperature and changes of phase between solid, liquid, and gas are traditionally considered part of chemistry, as are the gas laws. These kinds of changes in matter are called physical changes, because matter changes physical form but one substance does not change into a complete ...
Document
... The concept of temperature and changes of phase between solid, liquid, and gas are traditionally considered part of chemistry, as are the gas laws. These kinds of changes in matter are called physical changes, because matter changes physical form but one substance does not change into a complete ...
... The concept of temperature and changes of phase between solid, liquid, and gas are traditionally considered part of chemistry, as are the gas laws. These kinds of changes in matter are called physical changes, because matter changes physical form but one substance does not change into a complete ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.