Document
... The concept of temperature and changes of phase between solid, liquid, and gas are traditionally considered part of chemistry, as are the gas laws. These kinds of changes in matter are called physical changes, because matter changes physical form but one substance does not change into a complete ...
... The concept of temperature and changes of phase between solid, liquid, and gas are traditionally considered part of chemistry, as are the gas laws. These kinds of changes in matter are called physical changes, because matter changes physical form but one substance does not change into a complete ...
The Periodic Table HL Page 1 of 3 G. Galvin Name: Periodic Table
... Defn: Newland’s Octaves are arrangements of elements in which the first and the eighth element, counting from a particular element, have similar properties. 3. Mendeleev: Arranged the elements in order of increasing weight. Defn: Mendeleev’s Periodic Law: When elements are arranged in order of incre ...
... Defn: Newland’s Octaves are arrangements of elements in which the first and the eighth element, counting from a particular element, have similar properties. 3. Mendeleev: Arranged the elements in order of increasing weight. Defn: Mendeleev’s Periodic Law: When elements are arranged in order of incre ...
Regents questions
... Sample 7.1 Natural gas used in home heating and cooking is odorless. Because natural gas leaks pose the danger of explosion or suffocation, various smelly substances are added to the gas to allow detection of a leak. One such substance is methyl mercaptan, CH3SH. Use Figure 7.6 to predict the lengt ...
... Sample 7.1 Natural gas used in home heating and cooking is odorless. Because natural gas leaks pose the danger of explosion or suffocation, various smelly substances are added to the gas to allow detection of a leak. One such substance is methyl mercaptan, CH3SH. Use Figure 7.6 to predict the lengt ...
Stuff Matters Handout
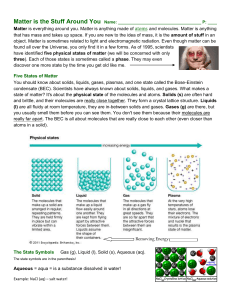
... Matter is everything around you. Matter is anything made of atoms and molecules. Matter is anything that has mass and takes up space. If you are new to the idea of mass, it is the amount of stuff in an object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can ...
... Matter is everything around you. Matter is anything made of atoms and molecules. Matter is anything that has mass and takes up space. If you are new to the idea of mass, it is the amount of stuff in an object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can ...
Instructor`s Notes Atomic Tiles: Play Your Way from Atoms to
... 3a. Students know the structure of the atom and know it is composed of protons, neutrons, and electrons. 3b. Students know that compounds are formed by combining two or more different elements and that compounds have properties that are different from their constituent elements. 5a. Students know re ...
... 3a. Students know the structure of the atom and know it is composed of protons, neutrons, and electrons. 3b. Students know that compounds are formed by combining two or more different elements and that compounds have properties that are different from their constituent elements. 5a. Students know re ...
PCSD General Chemistry Pacing Guide
... • Calculation of hydronium and hydroxide ions • Identification of common acids/bases ...
... • Calculation of hydronium and hydroxide ions • Identification of common acids/bases ...
matter
... • One material disperses evenly into another material so the first one seems to disappear Example: stirring sugar in tea ...
... • One material disperses evenly into another material so the first one seems to disappear Example: stirring sugar in tea ...
video slide
... a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom in a different molecule In living cells, the electronegative partners are usually oxygen or nitrogen atoms ...
... a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom in a different molecule In living cells, the electronegative partners are usually oxygen or nitrogen atoms ...
110 EXAM Review MATERIALTro
... b. Heterogeneous mixture has 2 or more physically distinct phases. Examples: ...
... b. Heterogeneous mixture has 2 or more physically distinct phases. Examples: ...
ChemicalBondingTestAnswers
... Dispersion forces are referred to as Vander Waals forces. 10. Substance IV is most likely to be an ionic compound as – it is solid in pure state, is highly soluble in water and has high solution conductivity. 11. HF and NH3 12. There are thousands of compounds that are uncommon or have multiple name ...
... Dispersion forces are referred to as Vander Waals forces. 10. Substance IV is most likely to be an ionic compound as – it is solid in pure state, is highly soluble in water and has high solution conductivity. 11. HF and NH3 12. There are thousands of compounds that are uncommon or have multiple name ...
Chapter 2
... Condensation cont. • This is an example of water and condensation but other substance act in the same way. ...
... Condensation cont. • This is an example of water and condensation but other substance act in the same way. ...
110 REVIEW MATERIALTro 2011
... b. Heterogeneous mixture has 2 or more physically distinct phases. Examples: ...
... b. Heterogeneous mixture has 2 or more physically distinct phases. Examples: ...
File - LSAmockscience
... • When one element replaces another element in a compound A + BC AC + B Element + compound new element + new compound ...
... • When one element replaces another element in a compound A + BC AC + B Element + compound new element + new compound ...
Unit 1 – Matter and Change
... – Made up of ONE type of Atom • Smallest unit of an element that maintains the chemical identity of that element • Smallest unit of matter with unique properties ...
... – Made up of ONE type of Atom • Smallest unit of an element that maintains the chemical identity of that element • Smallest unit of matter with unique properties ...
Chapter 2 Matter Study Guide
... 3. What makes up elements? A pure substance that cannot be broken down into any other substance 4. What makes up compounds? a substance made of two or more elements 5. What is a mixture? Made up of more than one kind of matter Can be separated physically by: Evaporation, Filtering, Sorting, Electric ...
... 3. What makes up elements? A pure substance that cannot be broken down into any other substance 4. What makes up compounds? a substance made of two or more elements 5. What is a mixture? Made up of more than one kind of matter Can be separated physically by: Evaporation, Filtering, Sorting, Electric ...
PART 2 – CHEMISTRY
... Let's look at why and how elements combine to form the molecules of every substance around us. If there are 2 electrons in a single shell surrounding the nucleus or 8 electrons in the outermost shell in the case where the atom has more than one shell, then the atom is said to be stable. This means t ...
... Let's look at why and how elements combine to form the molecules of every substance around us. If there are 2 electrons in a single shell surrounding the nucleus or 8 electrons in the outermost shell in the case where the atom has more than one shell, then the atom is said to be stable. This means t ...
Chapter 14 Chemical Reactions
... number of each type of atom on the product side and the reactant side. To balance the equation, we add another water molecule to the product side and add another oxygen molecule to the reactant side. ...
... number of each type of atom on the product side and the reactant side. To balance the equation, we add another water molecule to the product side and add another oxygen molecule to the reactant side. ...
5 - BrainMass
... smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy? b. Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron? Explain. ...
... smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy? b. Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron? Explain. ...
Atomic Weights Average Atomic Masses
... • Relative atomic mass: average masses of isotopes: – Naturally occurring C: 98.892 % 12C + 1.108 % 13C. • Average mass of C: • (0.98892)(12 amu) + (0.01108)(13.00335) = 12.011 amu. • Atomic weight (AW) is also known as average atomic mass (atomic weight). • Atomic weights are listed on the periodic ...
... • Relative atomic mass: average masses of isotopes: – Naturally occurring C: 98.892 % 12C + 1.108 % 13C. • Average mass of C: • (0.98892)(12 amu) + (0.01108)(13.00335) = 12.011 amu. • Atomic weight (AW) is also known as average atomic mass (atomic weight). • Atomic weights are listed on the periodic ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.