Atomic structure and periodic table
... There are over 100 elements so far discovered. Scientists have tried to group them together in a periodic table. A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Mosele ...
... There are over 100 elements so far discovered. Scientists have tried to group them together in a periodic table. A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Mosele ...
3. chemical bonding and molecular structure
... Ionic compounds dissolve in polar solvents like H2O due to ion-dipole interactions. Ionic compounds are generally insoluble in non-polar solvents like CHCl3, CCl4, CH3OH, C6H6, etc. Covalent bond: It was proposed by Lewis. The bond formed by sharing of electron pair is called covalent bond. In coval ...
... Ionic compounds dissolve in polar solvents like H2O due to ion-dipole interactions. Ionic compounds are generally insoluble in non-polar solvents like CHCl3, CCl4, CH3OH, C6H6, etc. Covalent bond: It was proposed by Lewis. The bond formed by sharing of electron pair is called covalent bond. In coval ...
Lab 1
... Primary substances, called elements, build all the materials about you. Some look similar, but others look unlike anything else. In this experiment, you will describe the physical properties of elements in a laboratory display and determine the location of elements on a blank periodic table. A. Phys ...
... Primary substances, called elements, build all the materials about you. Some look similar, but others look unlike anything else. In this experiment, you will describe the physical properties of elements in a laboratory display and determine the location of elements on a blank periodic table. A. Phys ...
Elements, Compounds, and Chemical Equations
... 2. Counting Atoms in Chemical Formulas: Examine the subscripts and coefficients • Subscripts describe the number of that type of atom. They appear after the element symbol, are small, and written hanging below the symbol. • Coefficients describe the number of molecules present. Coefficients apply t ...
... 2. Counting Atoms in Chemical Formulas: Examine the subscripts and coefficients • Subscripts describe the number of that type of atom. They appear after the element symbol, are small, and written hanging below the symbol. • Coefficients describe the number of molecules present. Coefficients apply t ...
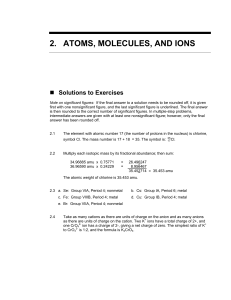
2.ATOMS, MOLECULES, AND IONS
... Some of the advantages of the Stock system are that more than two different ions of the same metal can be named with this system. In the former (older) system, a new suffix other than -ic and -ous must be established and/or memorized. ...
... Some of the advantages of the Stock system are that more than two different ions of the same metal can be named with this system. In the former (older) system, a new suffix other than -ic and -ous must be established and/or memorized. ...
LIQUIDS
... Definition: An atom is the smallest particle of an element that can exist or take part in a chemical change. MOLECULES All elements are made up of atoms. In some gaseous elements (e.g. argon) single atoms move around freely. But in other gaseous elements, single atoms cannot exist on their own at or ...
... Definition: An atom is the smallest particle of an element that can exist or take part in a chemical change. MOLECULES All elements are made up of atoms. In some gaseous elements (e.g. argon) single atoms move around freely. But in other gaseous elements, single atoms cannot exist on their own at or ...
Slide 1 ______
... Compound— When a molecule containing two or more different atoms forms Have characteristics different than the original atoms. ...
... Compound— When a molecule containing two or more different atoms forms Have characteristics different than the original atoms. ...
elements of chemistry unit
... Once the number and types of shared electrons has been determined, assign each shared electron to the more electronegative element. ELECTRONEGATIVITY An element’s ability to attract electrons is its electronegativity. In general, the halogens and group 16 atoms have the highest electronegativity. Th ...
... Once the number and types of shared electrons has been determined, assign each shared electron to the more electronegative element. ELECTRONEGATIVITY An element’s ability to attract electrons is its electronegativity. In general, the halogens and group 16 atoms have the highest electronegativity. Th ...
“Midterm” Exam # 1 - Elgin Community College
... 9) (4 pts) I have listed several temperatures below – CIRCLE the temperatures that are reasonable – CROSS OUT the bogus ones (one is right and one is wrong for each example) (the temperatures are approximate so if within a few degrees or so, it’s o.k.) 180°C or ...
... 9) (4 pts) I have listed several temperatures below – CIRCLE the temperatures that are reasonable – CROSS OUT the bogus ones (one is right and one is wrong for each example) (the temperatures are approximate so if within a few degrees or so, it’s o.k.) 180°C or ...
Chemistry - Set as Home Page
... 23. The symbolic representation of a molecule of a compound is called __________. 24. Molecular formula of CHCl3 and its Empirical formula is __________. 25. Molecular formula of benzene is C6H6 and its empirical formula is __________. 26. 58.5 is the __________ of NaCl. 27. 4.5 gms of nitrogen wil ...
... 23. The symbolic representation of a molecule of a compound is called __________. 24. Molecular formula of CHCl3 and its Empirical formula is __________. 25. Molecular formula of benzene is C6H6 and its empirical formula is __________. 26. 58.5 is the __________ of NaCl. 27. 4.5 gms of nitrogen wil ...
College Chemistry 1 Note Guide(free download)
... College Chemistry I is a course that covers the topics addressed in most first semester college chemistry courses. Many programs of study, particularly certain engineering degrees, require one semester of college chemistry as opposed to a two semester course, hence the year long course has been spli ...
... College Chemistry I is a course that covers the topics addressed in most first semester college chemistry courses. Many programs of study, particularly certain engineering degrees, require one semester of college chemistry as opposed to a two semester course, hence the year long course has been spli ...
Chemical Bonding
... a substance that you have classified as ionic, as it is an electrolyte. Like some other ionic compounds that you are familiar with, for example, baking soda (sodium hydrogen carbonate) and chalk (calcium carbonate), it is also brittle and has a high melting temperature. How do we explain the formati ...
... a substance that you have classified as ionic, as it is an electrolyte. Like some other ionic compounds that you are familiar with, for example, baking soda (sodium hydrogen carbonate) and chalk (calcium carbonate), it is also brittle and has a high melting temperature. How do we explain the formati ...
chapter2
... • An example of an isotope symbol is 28 Ni. This symbol represents an isotope of nickel that contains 28 protons and 32 neutrons in the nucleus. • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of thi ...
... • An example of an isotope symbol is 28 Ni. This symbol represents an isotope of nickel that contains 28 protons and 32 neutrons in the nucleus. • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of thi ...
Final
... 26. 5 pts. Identify the oxidizing agent, the reducing agent, the element being oxidized, the element being reduced, and the number of electrons transferred in this balanced chemical equation: 4 HNO3 + 3 S Æ 3 SO2 + 4 NO + 2 H2O oxidizing agent ______________________ atom being oxidized _____ ...
... 26. 5 pts. Identify the oxidizing agent, the reducing agent, the element being oxidized, the element being reduced, and the number of electrons transferred in this balanced chemical equation: 4 HNO3 + 3 S Æ 3 SO2 + 4 NO + 2 H2O oxidizing agent ______________________ atom being oxidized _____ ...
Balancing Reaction Equations Oxidation State Reduction
... Determining Oxidation Number of Elements & Compounds 1. In uncombined or free elements (not ionized), each atom has an oxidation number of 0. E.g., all of the atoms in these molecules: H2, Na, S8, O2, P4. 2. In simple ions (i.e., charged species which contain only one atom), the oxidation number ...
... Determining Oxidation Number of Elements & Compounds 1. In uncombined or free elements (not ionized), each atom has an oxidation number of 0. E.g., all of the atoms in these molecules: H2, Na, S8, O2, P4. 2. In simple ions (i.e., charged species which contain only one atom), the oxidation number ...
AP Chemistry - Oak Park Unified School District
... Science knowledge is advanced by observing patterns, (1), and constructing explanations, (2); which are supported by repeatable (3) evidence. Measurements are made using the metric system, where the standard units are called (4) units, which are based on the meter, kilogram, and second as the basic ...
... Science knowledge is advanced by observing patterns, (1), and constructing explanations, (2); which are supported by repeatable (3) evidence. Measurements are made using the metric system, where the standard units are called (4) units, which are based on the meter, kilogram, and second as the basic ...
PHYSICAL SETTING CHEMISTRY
... the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure ...
... the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure ...
Atoms and bonds in molecules and chemical
... or principle. The unification approach intends to derive the occurrence of the event using a theory that unifies many phenomena or the theory that unifies the phenomena better than any other. In the causal model the explanation will trace the causal processes and interactions leading to the event (i ...
... or principle. The unification approach intends to derive the occurrence of the event using a theory that unifies many phenomena or the theory that unifies the phenomena better than any other. In the causal model the explanation will trace the causal processes and interactions leading to the event (i ...
elements of chemistry unit
... LDS diagrams are not always needed to predict oxidation numbers. As shown above, the four rules of oxidation can also be used to predict oxidation numbers. Example 4. Use the rules of oxidation to predict the oxidation numbers for carbon and oxygen within the carbon dioxide molecule. 4A. The molecul ...
... LDS diagrams are not always needed to predict oxidation numbers. As shown above, the four rules of oxidation can also be used to predict oxidation numbers. Example 4. Use the rules of oxidation to predict the oxidation numbers for carbon and oxygen within the carbon dioxide molecule. 4A. The molecul ...
10/18/11 - Note: Once it is downloaded, click SET
... What’s involved? Periodic table, electron, atomic number Electrons are arranged in orbitals around the nucleus Things to know: -Hund’s Rule, Aufbau Principle, Pauli’s Exclusion Principle -Electron Dot- shows how many valence electrons it has. -SPDF (orbitals) S- 1- up to 2 electrons P- 3- up to 6 el ...
... What’s involved? Periodic table, electron, atomic number Electrons are arranged in orbitals around the nucleus Things to know: -Hund’s Rule, Aufbau Principle, Pauli’s Exclusion Principle -Electron Dot- shows how many valence electrons it has. -SPDF (orbitals) S- 1- up to 2 electrons P- 3- up to 6 el ...
Openstax - Chemistry - Answer Key
... 3. This statement violates Dalton’s fourth postulate: In a given compound, the numbers of atoms of each type (and thus also the percentage) always have the same ratio. 5. Dalton originally thought that all atoms of a particular element had identical properties, including mass. Thus, the concept of i ...
... 3. This statement violates Dalton’s fourth postulate: In a given compound, the numbers of atoms of each type (and thus also the percentage) always have the same ratio. 5. Dalton originally thought that all atoms of a particular element had identical properties, including mass. Thus, the concept of i ...
Chemistry - Textbooks Online
... Rutherford suggested the planetary model, but this model was rejected. ...
... Rutherford suggested the planetary model, but this model was rejected. ...
AP Chemistry
... state, otherwise it would go into the 2s sublevel. 1s() 2s() 2p()()(): The 2 2s electrons and 3 2p electrons are in the valence shell (highest energy level) five. 1s(): The first ionized electron is from the 1s sublevel. It takes the most energy to remove electrons that are close to ...
... state, otherwise it would go into the 2s sublevel. 1s() 2s() 2p()()(): The 2 2s electrons and 3 2p electrons are in the valence shell (highest energy level) five. 1s(): The first ionized electron is from the 1s sublevel. It takes the most energy to remove electrons that are close to ...
Chapter 2 power point File
... Scales are used by scientists to measure samples and to find out specific information A meter scale measures things that are large (length of a car), a centimeter measures things that are small (the length of a book), and a millimeter measures things that are microscopic or really small (like the th ...
... Scales are used by scientists to measure samples and to find out specific information A meter scale measures things that are large (length of a car), a centimeter measures things that are small (the length of a book), and a millimeter measures things that are microscopic or really small (like the th ...
PHYSICAL SETTING CHEMISTRY
... The possession or use of any communications device is strictly prohibited when taking this examination. If you have or use any communications device, no matter how briefly, your examination will be invalidated and no score will be calculated for you. This is a test of your knowledge of chemistry. Us ...
... The possession or use of any communications device is strictly prohibited when taking this examination. If you have or use any communications device, no matter how briefly, your examination will be invalidated and no score will be calculated for you. This is a test of your knowledge of chemistry. Us ...
Electronegativity

Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. The term ""electronegativity"" was introduced by Jöns Jacob Berzelius in 1811,though the concept was known even before that and was studied by many chemists including Avogadro.In spite of its long history, an accurate scale of electronegativity had to wait till 1932, when Linus Pauling proposed an electronegativity scale, which depends on bond energies, as a development of valence bond theory. It has been shown to correlate with a number of other chemical properties. Electronegativity cannot be directly measured and must be calculated from other atomic or molecular properties. Several methods of calculation have been proposed, and although there may be small differences in the numerical values of the electronegativity, all methods show the same periodic trends between elements. The most commonly used method of calculation is that originally proposed by Linus Pauling. This gives a dimensionless quantity, commonly referred to as the Pauling scale, on a relative scale running from around 0.7 to 3.98 (hydrogen = 2.20). When other methods of calculation are used, it is conventional (although not obligatory) to quote the results on a scale that covers the same range of numerical values: this is known as an electronegativity in Pauling units. As it is usually calculated, electronegativity is not a property of an atom alone, but rather a property of an atom in a molecule. Properties of a free atom include ionization energy and electron affinity. It is to be expected that the electronegativity of an element will vary with its chemical environment, but it is usually considered to be a transferable property, that is to say that similar values will be valid in a variety of situations.On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more ""pull"" it will have on electrons) and the number/location of other electrons present in the atomic shells (the more electrons an atom has, the farther from the nucleus the valence electrons will be, and as a result the less positive charge they will experience—both because of their increased distance from the nucleus, and because the other electrons in the lower energy core orbitals will act to shield the valence electrons from the positively charged nucleus).The opposite of electronegativity is electropositivity: a measure of an element's ability to donate electrons.Caesium is the least electronegative element in the periodic table (=0.79), while fluorine is most electronegative (=3.98). (Francium and caesium were originally assigned both assigned 0.7; caesium's value was later refined to 0.79, but no experimental data allows a similar refinement for francium. However, francium's ionization energy is known to be slightly higher than caesium's, in accordance with the relativistic stabilization of the 7s orbital, and this in turn implies that caesium is in fact more electronegative than francium.)