Document
... Electromagnetic Energy Niels Bohr proposed in 1914 a model of the hydrogen atom as a nucleus with an electron circling around it. In this model, the energy levels of the orbits are quantized so that only certain specific orbits corresponding to certain specific energies for the electron are availabl ...
... Electromagnetic Energy Niels Bohr proposed in 1914 a model of the hydrogen atom as a nucleus with an electron circling around it. In this model, the energy levels of the orbits are quantized so that only certain specific orbits corresponding to certain specific energies for the electron are availabl ...
Answers to Selected Problems
... 45. protons and neutrons 47. the number of protons in the nucleus 49. The atomic number is the number of protons. ...
... 45. protons and neutrons 47. the number of protons in the nucleus 49. The atomic number is the number of protons. ...
Second-order coupling between excited atoms and surface polaritons
... In this article, we analyze a new type of near-field effect involving surface polaritons inspired by the experiment of Kübler et al. [13] with hot Rb vapors in glass cells. Their experiment indicated that a description of the atom-surface interactions should also include a second-order coupling bet ...
... In this article, we analyze a new type of near-field effect involving surface polaritons inspired by the experiment of Kübler et al. [13] with hot Rb vapors in glass cells. Their experiment indicated that a description of the atom-surface interactions should also include a second-order coupling bet ...
dicke-july2013x
... OK for fixed atoms, but I said we’d consider motion! • We’ve incorporated CoM coordinates into , the “cooperation” operator; does not commute with ! • Thus, these are not stationary eigenstates of . • Classically, relative motion of radiators causes decoherence, but radiators with a common velocity ...
... OK for fixed atoms, but I said we’d consider motion! • We’ve incorporated CoM coordinates into , the “cooperation” operator; does not commute with ! • Thus, these are not stationary eigenstates of . • Classically, relative motion of radiators causes decoherence, but radiators with a common velocity ...
Additional Review
... o all of matter is some combination of these four elements Alchemy [1500 AD] In the 1500’s many scientists were________________________________________________ ________________________________________________________________________________________ While they were not able to create gold they did di ...
... o all of matter is some combination of these four elements Alchemy [1500 AD] In the 1500’s many scientists were________________________________________________ ________________________________________________________________________________________ While they were not able to create gold they did di ...
Section 11.3 Atomic Orbitals
... Atoms Beyond Hydrogen • Pauli Exclusion Principle - No 2electrons in the same atom can have the same set of 4 quantum numbers. An atomic orbital can hold a maximum of 2 electrons and those 2 electrons must have opposite spins • Hund’s Rule – every orbital in a subshell is singly occupied with one el ...
... Atoms Beyond Hydrogen • Pauli Exclusion Principle - No 2electrons in the same atom can have the same set of 4 quantum numbers. An atomic orbital can hold a maximum of 2 electrons and those 2 electrons must have opposite spins • Hund’s Rule – every orbital in a subshell is singly occupied with one el ...
Atom-Light Interactions - Durham University Community
... by the wavefunction Ψbeam ; in fact it cannot be described by a wavefunction at all. This type of state is called a mixed state. Note that this mixed state would still give a click 50% of the time on average if we were to repeat the measurement further downstream. How do we distinguish therefore bet ...
... by the wavefunction Ψbeam ; in fact it cannot be described by a wavefunction at all. This type of state is called a mixed state. Note that this mixed state would still give a click 50% of the time on average if we were to repeat the measurement further downstream. How do we distinguish therefore bet ...
Phys405-Chapter5
... connected to both a temperature controller and a second controller for the injection current of the diode. Light from the diode laser is collimated and attenuated before exiting the laser safety box. The laser light then passes through a cell filled with natural rubidium and is detected by a photodi ...
... connected to both a temperature controller and a second controller for the injection current of the diode. Light from the diode laser is collimated and attenuated before exiting the laser safety box. The laser light then passes through a cell filled with natural rubidium and is detected by a photodi ...
Lecture 1 Atomic Structure
... electrons and a positively charged part, but it was not clear how atoms are constructed. In 1898, J.J. Thomson suggested that an atom might be a positively charged sphere in which negatively charged electrons are embedded. This model of the atom is sometimes called the “plum pudding” model. Of cours ...
... electrons and a positively charged part, but it was not clear how atoms are constructed. In 1898, J.J. Thomson suggested that an atom might be a positively charged sphere in which negatively charged electrons are embedded. This model of the atom is sometimes called the “plum pudding” model. Of cours ...
Introductory Chemistry I
... 4. The maximum number of electrons that can occupy the 3d orbitals is a. 5 b. 6 c. 10 d. 14 e. 18 5. Let’s say that you are examining the outermost electrons in a ground-state germanium atom. Which of the following sets of values for the four quantum numbers (n, l, ml, and ms) could you use to descr ...
... 4. The maximum number of electrons that can occupy the 3d orbitals is a. 5 b. 6 c. 10 d. 14 e. 18 5. Let’s say that you are examining the outermost electrons in a ground-state germanium atom. Which of the following sets of values for the four quantum numbers (n, l, ml, and ms) could you use to descr ...
Chapter 2 Rydberg Atoms
... the alkali metal atoms, the interaction with the core creates a perturbation to the hydrogenic states that is characterised by the quantum defects. Using a model potential, the wavefunctions can be obtained numerically, enabling calculation of the transition dipole matrix elements between the states ...
... the alkali metal atoms, the interaction with the core creates a perturbation to the hydrogenic states that is characterised by the quantum defects. Using a model potential, the wavefunctions can be obtained numerically, enabling calculation of the transition dipole matrix elements between the states ...
Monday, Oct. 2, 2006
... Nuclear Models: Shell Model • Exploit the success of atomic model – Uses orbital structure of nucleons – Electron energy levels are quantized – Limited number of electrons in each level based on available spin and angular momentum configurations • For nth energy level, l angular momentum (l
... Nuclear Models: Shell Model • Exploit the success of atomic model – Uses orbital structure of nucleons – Electron energy levels are quantized – Limited number of electrons in each level based on available spin and angular momentum configurations • For nth energy level, l angular momentum (l
atom-ph/9606004 PDF
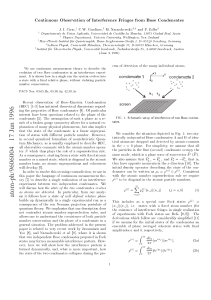
... Recent observation of Bose{Einstein Condensation (BEC) [1{3] has initiated theoretical discussions regarding the properties of Bose condensates [4]. Of particular interest have been questions related to the phase of the condensate [5]. The assumption of such a phase as a result of a broken gauge sym ...
... Recent observation of Bose{Einstein Condensation (BEC) [1{3] has initiated theoretical discussions regarding the properties of Bose condensates [4]. Of particular interest have been questions related to the phase of the condensate [5]. The assumption of such a phase as a result of a broken gauge sym ...