PPT - mvhs-fuhsd.org
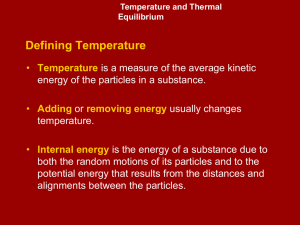
... • Energy is the ability to do work or transfer heat. – Energy used to cause an object that has mass to move is called work. – Energy used to cause the temperature of an object to rise is called heat. – There are different forms of energies- radiant, thermal, chemical, potential etc. We will focus on ...
... • Energy is the ability to do work or transfer heat. – Energy used to cause an object that has mass to move is called work. – Energy used to cause the temperature of an object to rise is called heat. – There are different forms of energies- radiant, thermal, chemical, potential etc. We will focus on ...
exam2gc1sum11+key
... _____7. What is the oxidation number of N in NaNO2? A. -1 B. 3 _____8. Iron is more active than silver but less active than zinc. Which one of the following reactions will occur? A. Fe + 2Ag+ Fe+2 + 2Ag C. 3Ag + Fe+3 3Ag+ + Fe B. Fe + Zn+2Fe+2 + Zn D. Zn+2 + 2Ag Zn + 2Ag+ _____9. Based on the s ...
... _____7. What is the oxidation number of N in NaNO2? A. -1 B. 3 _____8. Iron is more active than silver but less active than zinc. Which one of the following reactions will occur? A. Fe + 2Ag+ Fe+2 + 2Ag C. 3Ag + Fe+3 3Ag+ + Fe B. Fe + Zn+2Fe+2 + Zn D. Zn+2 + 2Ag Zn + 2Ag+ _____9. Based on the s ...
an improved heat soak calculation for mechanical seals
... heat soak from viscous process fluids and buffer/barrier fluids. In general, heat soak to fluids more viscous than water will be less than the default API estimate for heat soak. Except for viscosity, all other fluid properties can be combined into the fluid property factor, m6. This factor includes ...
... heat soak from viscous process fluids and buffer/barrier fluids. In general, heat soak to fluids more viscous than water will be less than the default API estimate for heat soak. Except for viscosity, all other fluid properties can be combined into the fluid property factor, m6. This factor includes ...
Maxwell Relations
... Helmholtz Free Energy dF d (U TS ) SdT PdV Gibb' s Function dG d ( F PV ) SdT VdP ...
... Helmholtz Free Energy dF d (U TS ) SdT PdV Gibb' s Function dG d ( F PV ) SdT VdP ...
Chapter Two The Thermodynamic Laws
... No matter how much heat is delivered, temperature of the reservoir will never change. There are many statements of the second law which use different terms, but are all equivalent. (2.3.2.2). Kelvin-Plank Statement It is impossible for any system to operate in a thermodynamic cycle and deliver a net ...
... No matter how much heat is delivered, temperature of the reservoir will never change. There are many statements of the second law which use different terms, but are all equivalent. (2.3.2.2). Kelvin-Plank Statement It is impossible for any system to operate in a thermodynamic cycle and deliver a net ...
File
... there is a change in potential energy. We cannot directly measure the change in potential energy that occurs during the chemical reaction. Potential energy can be converted into kinetic energy. The energy change of the chemical reaction or a physical change of state affects the surroundings (the env ...
... there is a change in potential energy. We cannot directly measure the change in potential energy that occurs during the chemical reaction. Potential energy can be converted into kinetic energy. The energy change of the chemical reaction or a physical change of state affects the surroundings (the env ...
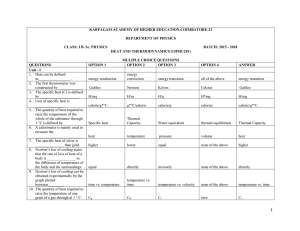
Heat and Thermodynamics 300 MCQ
... weights of all gases occupy the same volume is known as 47. If a gas is heated against a pressure keeping the volume constant, then work done will be equal to 48. Thermal conduction in metals take place by 49. If c is the length and A area of cross section of a rod and k is thermal conductivity of m ...
... weights of all gases occupy the same volume is known as 47. If a gas is heated against a pressure keeping the volume constant, then work done will be equal to 48. Thermal conduction in metals take place by 49. If c is the length and A area of cross section of a rod and k is thermal conductivity of m ...
Energy
... Energy is the capacity to do work or transfer heat. In principal any kind of energy can be converted into an equivalent amount of work or heat. We divide energy into two general types: kinetic energy (due to motion in a particular direction) EK = 1/2 mv2 potential energy (due to position or composit ...
... Energy is the capacity to do work or transfer heat. In principal any kind of energy can be converted into an equivalent amount of work or heat. We divide energy into two general types: kinetic energy (due to motion in a particular direction) EK = 1/2 mv2 potential energy (due to position or composit ...
An availability approach to thermal energy recovery in vehicles
... The failure of practical engines to approach a high rational efficiency is due to a range of irreversible processes. Within the cylinder, expansion of gases is rapid, with consequent large temperature differences, fluid motion, and heat transfer to the cylinder walls and piston crown. In order for the c ...
... The failure of practical engines to approach a high rational efficiency is due to a range of irreversible processes. Within the cylinder, expansion of gases is rapid, with consequent large temperature differences, fluid motion, and heat transfer to the cylinder walls and piston crown. In order for the c ...
PREPARMACY PHYSICAL CHEMISTRY THERMOCHEMISTRY
... ammonium hydroxide is dissociated into ions. In general, the heat of dissociation of a weak acid or weak base may be defined as the change in enthalpy of the system when one mole of it is dissociated into ions. ...
... ammonium hydroxide is dissociated into ions. In general, the heat of dissociation of a weak acid or weak base may be defined as the change in enthalpy of the system when one mole of it is dissociated into ions. ...
Powerpoint
... Enthalpy(焓) Enthalpy (H) is the heat content of a substance. It can also be considered as the total energy of a substance. When a physical process or chemical reaction occurs, heat is usually given out or absorbed from the surroundings. e.g., ice melting, condensation of water vapor, burning of coa ...
... Enthalpy(焓) Enthalpy (H) is the heat content of a substance. It can also be considered as the total energy of a substance. When a physical process or chemical reaction occurs, heat is usually given out or absorbed from the surroundings. e.g., ice melting, condensation of water vapor, burning of coa ...
Chapter 5 Thermochemistry
... SAMPLE EXERCISE 5.2 Relating heat and work to changes of internal energy Two gases, A(g) and B(g), are confined in a cylinder-and-piston arrangement like that in Figure 5.3. Substances A and B react to form a solid product: As the reactions occurs, the system loses 1150 J of heat to the surrounding. ...
... SAMPLE EXERCISE 5.2 Relating heat and work to changes of internal energy Two gases, A(g) and B(g), are confined in a cylinder-and-piston arrangement like that in Figure 5.3. Substances A and B react to form a solid product: As the reactions occurs, the system loses 1150 J of heat to the surrounding. ...
b - UCSC Physics
... The latent heat of vaporization is relevant for evaporation as well as boiling. The heat of vaporization of water rises slightly as the temperature decreases. On a molecular level, the heat added during a change of state does not go to increasing the kinetic energy of individual molecules, but rathe ...
... The latent heat of vaporization is relevant for evaporation as well as boiling. The heat of vaporization of water rises slightly as the temperature decreases. On a molecular level, the heat added during a change of state does not go to increasing the kinetic energy of individual molecules, but rathe ...
chemistry mcmurry fay
... motion and is measured by finding the temperature of an object. (including rotational and vibrational) Thermal energy is proportional to the temperature in degrees Kelvin. Ethermal T (oK) Heat is the amount of thermal energy transferred between two objects at different temperatures. Heat involves ...
... motion and is measured by finding the temperature of an object. (including rotational and vibrational) Thermal energy is proportional to the temperature in degrees Kelvin. Ethermal T (oK) Heat is the amount of thermal energy transferred between two objects at different temperatures. Heat involves ...
Heat transfer

Heat transfer is the exchange of thermal energy between physical systems, depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation.Heat transfer always occurs from a region of high temperature to another region of lower temperature. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. The Second Law of Thermodynamics defines the concept of thermodynamic entropy, by measurable heat transfer.Thermal equilibrium is reached when all involved bodies and the surroundings reach the same temperature. Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.