1990-Spring-Exam-2-student
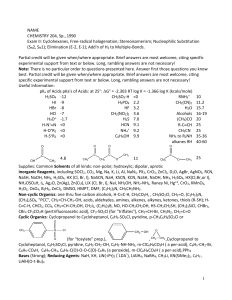
... experimental support from text or below. Long, rambling answers are not necessary! Note: There is no particular order to questions presented here. Answer first those questions you know best. Partial credit will be given when/where appropriate. Brief answers are most welcome, citing specific experime ...
... experimental support from text or below. Long, rambling answers are not necessary! Note: There is no particular order to questions presented here. Answer first those questions you know best. Partial credit will be given when/where appropriate. Brief answers are most welcome, citing specific experime ...
Chapter 9 Lota_2 Dæmi A4 Varmafræði
... The specific heat of gold is 0.13 J g–1 K–1, and that of copper is 0.39 J g–1 K–1. Suppose that we heat both a 25-g sample of gold and a 25-g sample of copper to 80°C and then drop each into identical beakers containing 100 mL of cold water at 10°C. When each beaker reaches thermal equilibrium, whic ...
... The specific heat of gold is 0.13 J g–1 K–1, and that of copper is 0.39 J g–1 K–1. Suppose that we heat both a 25-g sample of gold and a 25-g sample of copper to 80°C and then drop each into identical beakers containing 100 mL of cold water at 10°C. When each beaker reaches thermal equilibrium, whic ...
AddCorrections(KKH) - Spiral
... Traditionally, there are three general ways of converting alkenes into alcohols. The first route requires protonation of the C=C bond to form a carbocation intermediate, which is then attacked by water to give an alcohol (Scheme 1, eq. 1). Selective formation of the thermodynamically-preferred carbo ...
... Traditionally, there are three general ways of converting alkenes into alcohols. The first route requires protonation of the C=C bond to form a carbocation intermediate, which is then attacked by water to give an alcohol (Scheme 1, eq. 1). Selective formation of the thermodynamically-preferred carbo ...
Cl + CH3OH * HCl + CH2OH
... A rough estimate for a generic uptake coefficient for NO3 uptake to saturated alcohols or carbonyls can be made using kb = 6 104 M-1 s-1 (equivalent to a gas-phase rate constant of 1 10-16 cm3 molecule-1 s-1), Dl, = 2 10-5 cm2 s-1, and H = 0.8 Matm-1, this expression results in a value of = ...
... A rough estimate for a generic uptake coefficient for NO3 uptake to saturated alcohols or carbonyls can be made using kb = 6 104 M-1 s-1 (equivalent to a gas-phase rate constant of 1 10-16 cm3 molecule-1 s-1), Dl, = 2 10-5 cm2 s-1, and H = 0.8 Matm-1, this expression results in a value of = ...
equilibrium - TeacherWeb
... The direction in which you write the chemical equation for an equilibrium is arbitrary, because equilibrium can be approached from either direction. The equilibrium constant expression for a reaction written in one direction is the reciprocal of the one for the reaction in the reverse direction. The ...
... The direction in which you write the chemical equation for an equilibrium is arbitrary, because equilibrium can be approached from either direction. The equilibrium constant expression for a reaction written in one direction is the reciprocal of the one for the reaction in the reverse direction. The ...
18.3 Standard Entropies and the Third Law of
... CHAPTER TERMS AND DEFINITIONS Numbers in parentheses after definitions give the text section in which the terms are explained. Starred terms are italicized in the text. Where a term does not fall directly under a text section heading, additional information is given for you to locate it. thermodynam ...
... CHAPTER TERMS AND DEFINITIONS Numbers in parentheses after definitions give the text section in which the terms are explained. Starred terms are italicized in the text. Where a term does not fall directly under a text section heading, additional information is given for you to locate it. thermodynam ...
Boston University Dresden Science Program Instructor: Meeting Times
... All personal possessions are to be placed at the front of the room. Hats with visors are to be turned backwards. There is no talking to other students or sharing of any materials such as molecular models. If you need to go to the restroom you must be accompanied by a teaching fellow. You may not sta ...
... All personal possessions are to be placed at the front of the room. Hats with visors are to be turned backwards. There is no talking to other students or sharing of any materials such as molecular models. If you need to go to the restroom you must be accompanied by a teaching fellow. You may not sta ...
Chemistry Higher Level Chapter 5 - Pearson Schools and FE Colleges
... of knowledge? Is their correct use a necessary or sufficient indicator of understanding? ...
... of knowledge? Is their correct use a necessary or sufficient indicator of understanding? ...
Synthesis of enantiopure alcohols
... Synthesis of enantiopure alcohols (R)-1-Phenoxy-2-butanol ((R)-1) The butanoate of 1-phenoxy-2-butanol, 1a, (0.8691g, 3.68 mmole) was hydrolyzed by addition of CAL-B Novozym 435 (0.105 g) in phosphate buffer (0.1 M, 183.5 mL). The enantiopure alcohol (R)-1 was separated from the remaining butanoate ...
... Synthesis of enantiopure alcohols (R)-1-Phenoxy-2-butanol ((R)-1) The butanoate of 1-phenoxy-2-butanol, 1a, (0.8691g, 3.68 mmole) was hydrolyzed by addition of CAL-B Novozym 435 (0.105 g) in phosphate buffer (0.1 M, 183.5 mL). The enantiopure alcohol (R)-1 was separated from the remaining butanoate ...
5 Energetics - Pearson Schools and FE Colleges
... of knowledge? Is their correct use a necessary or sufficient indicator of understanding? ...
... of knowledge? Is their correct use a necessary or sufficient indicator of understanding? ...
121. Acceptorless Dehydrogenation with Metal
... can be as high as 0.1%. Scope of the substrates are quite large. Aryl, alkyl, alkeyl(some examples) are both appropriate substituent groups of the substrates. Detailed studied about the operation pattern of catalysts will be a new orientation in modern chemistry. ...
... can be as high as 0.1%. Scope of the substrates are quite large. Aryl, alkyl, alkeyl(some examples) are both appropriate substituent groups of the substrates. Detailed studied about the operation pattern of catalysts will be a new orientation in modern chemistry. ...