Core organic chemistry
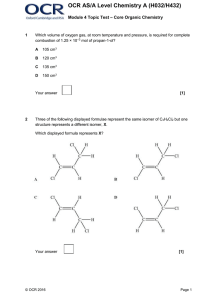
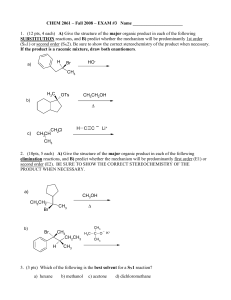
... The elimination of H2O from pentan-2-ol forms a mixture of organic products. Give the names and structures of all the organic products in the mixture. Your answer should explain how the reaction leads to the different isomers. ...
... The elimination of H2O from pentan-2-ol forms a mixture of organic products. Give the names and structures of all the organic products in the mixture. Your answer should explain how the reaction leads to the different isomers. ...
Ir-catalysed formation of C− F bonds. From allylic alcohols to α
... a Z1-mode through the oxygen atom (IIa) or through the methylenic carbon (IIc), or in a Z3-mode as an oxaallyl (IIb).15b,19 In step (iii), the C–F bond is formed upon reaction with the electrophilic SelectF. Step (iii) can occur directly from II, or via formation of the free enol.y In conclusion, we ...
... a Z1-mode through the oxygen atom (IIa) or through the methylenic carbon (IIc), or in a Z3-mode as an oxaallyl (IIb).15b,19 In step (iii), the C–F bond is formed upon reaction with the electrophilic SelectF. Step (iii) can occur directly from II, or via formation of the free enol.y In conclusion, we ...
Basic Stoichometry
... Imagine if you made a batch of cookies and used way too many eggs, or not enough sugar. YUCK! In chemistry, reactions proceed with very specific recipes. The study of these recipes is stoichiometry. When the reactants are present in the correct amounts, the reaction will produce products. What happe ...
... Imagine if you made a batch of cookies and used way too many eggs, or not enough sugar. YUCK! In chemistry, reactions proceed with very specific recipes. The study of these recipes is stoichiometry. When the reactants are present in the correct amounts, the reaction will produce products. What happe ...
Chemistry Syllabus - Eleanor Roosevelt High School
... What are the different forms of energy and how is energy conserved and converted into matter? How do we explain the behavior of radioactive isotopes and how can they beneficial in medicine, dating of materials, nuclear power, and industry? How do the Laws of Thermodynamics and the concept of entropy ...
... What are the different forms of energy and how is energy conserved and converted into matter? How do we explain the behavior of radioactive isotopes and how can they beneficial in medicine, dating of materials, nuclear power, and industry? How do the Laws of Thermodynamics and the concept of entropy ...
4 Expressing and Measuring Chemical Change
... occurs remains constant. Priestley and Lavoisier were both radical thinkers for the time they lived in. Englishman Priestley’s support of the French Revolutionists led to his persecution, and he eventually had to flee from Europe altogether. He lived the last of his days in Pennsylvania in the Unite ...
... occurs remains constant. Priestley and Lavoisier were both radical thinkers for the time they lived in. Englishman Priestley’s support of the French Revolutionists led to his persecution, and he eventually had to flee from Europe altogether. He lived the last of his days in Pennsylvania in the Unite ...
Document
... products and reactants at equilibrium. • Kinetics determines the pathway by which equilibrium is reached. • A high activation energy can effectively block a reaction that is thermodynamically favored. • Example: combustion reactions are thermodynamically favored, but (fortunately for life on Earth!) ...
... products and reactants at equilibrium. • Kinetics determines the pathway by which equilibrium is reached. • A high activation energy can effectively block a reaction that is thermodynamically favored. • Example: combustion reactions are thermodynamically favored, but (fortunately for life on Earth!) ...
19 BROWN Chemical Thermodynamics PPTSExercise
... The temperature dependence of ΔG° comes from the entropy term. We expect ΔS° for this reaction to be negative because the number of molecules of gas is smaller in the products. Because ΔS° is negative, the term –T ΔS° is positive and grows larger with increasing temperature. As a result, ΔG° becomes ...
... The temperature dependence of ΔG° comes from the entropy term. We expect ΔS° for this reaction to be negative because the number of molecules of gas is smaller in the products. Because ΔS° is negative, the term –T ΔS° is positive and grows larger with increasing temperature. As a result, ΔG° becomes ...
Slide 1
... The temperature dependence of ΔG° comes from the entropy term. We expect ΔS° for this reaction to be negative because the number of molecules of gas is smaller in the products. Because ΔS° is negative, the term –T ΔS° is positive and grows larger with increasing temperature. As a result, ΔG° becomes ...
... The temperature dependence of ΔG° comes from the entropy term. We expect ΔS° for this reaction to be negative because the number of molecules of gas is smaller in the products. Because ΔS° is negative, the term –T ΔS° is positive and grows larger with increasing temperature. As a result, ΔG° becomes ...
Microsoft Word - Open Access Repository of Indian Theses
... alcohols in a regioselective manner with preferential nucleophilic attack at benzylic position. Aliphatic amines on reaction with styrene oxide gave the product with opposite regiochemistry. The methodology was further extended to the ring opening of aliphatic epoxides and substituted styrene oxide ...
... alcohols in a regioselective manner with preferential nucleophilic attack at benzylic position. Aliphatic amines on reaction with styrene oxide gave the product with opposite regiochemistry. The methodology was further extended to the ring opening of aliphatic epoxides and substituted styrene oxide ...