Document
... cations, it is possible to generate a huge number of different ionic liquids, each with their own specific solvent properties. Some ionic liquids are water soluble, others are not. Some dissolve typical organic solvents, other are not. • They can be functionalized to act as acids, bases or ligands a ...
... cations, it is possible to generate a huge number of different ionic liquids, each with their own specific solvent properties. Some ionic liquids are water soluble, others are not. Some dissolve typical organic solvents, other are not. • They can be functionalized to act as acids, bases or ligands a ...
Amines
... Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on the substituents present on the original amide. Look at the N substitu ...
... Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on the substituents present on the original amide. Look at the N substitu ...
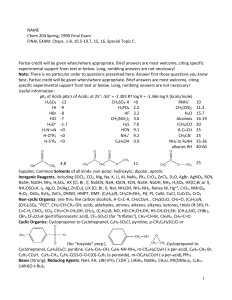
NAME Chem 204 Spring, 1990 Final Exam FINAL EXAM: Chaps. 1
... alkyl chloride, there is NO one reaction that will convert a chiral alcohol into its alkyl chloride with "retained" configuration. Thus, it is necessary to use several reaction steps, each of 100% inversion or 100% retention of configuration, in order to convert (X) into (XI). Outlines below a serie ...
... alkyl chloride, there is NO one reaction that will convert a chiral alcohol into its alkyl chloride with "retained" configuration. Thus, it is necessary to use several reaction steps, each of 100% inversion or 100% retention of configuration, in order to convert (X) into (XI). Outlines below a serie ...
M.Sc. Part-I Chemistry - North Maharashtra University
... and sandals are prohibited. Long hair must be tied up. Each student will have to get his / her own necessary protection items. 3) Prior to the practical examination, the teacher-in-cvharge will check all protective equipment to ensure that they are in order. 4) Pipetting by mouth should be avoided. ...
... and sandals are prohibited. Long hair must be tied up. Each student will have to get his / her own necessary protection items. 3) Prior to the practical examination, the teacher-in-cvharge will check all protective equipment to ensure that they are in order. 4) Pipetting by mouth should be avoided. ...
updated chem cp final review key
... f. Adding an enzyme Increases rate of reaction g. Breaking a reactant into smaller pieces Increases rate of reaction 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop ...
... f. Adding an enzyme Increases rate of reaction g. Breaking a reactant into smaller pieces Increases rate of reaction 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop ...
ALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO …
... •At the end of the reaction, because imines come apart easily (remember the “unfavorable” equilibrium?), the modified substrate can dissociate from the enzyme and return to the solution. •As we can see, often biological substrates possess carbonyl groups so that they can act as a “handle” in enzyme- ...
... •At the end of the reaction, because imines come apart easily (remember the “unfavorable” equilibrium?), the modified substrate can dissociate from the enzyme and return to the solution. •As we can see, often biological substrates possess carbonyl groups so that they can act as a “handle” in enzyme- ...
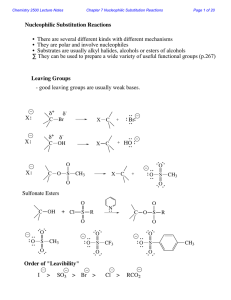
Nucleophilic Substitution Reactions
... With a charged nucleophile (i.e. Nu: = HO or NC ) and a leaving group, LG H ...
... With a charged nucleophile (i.e. Nu: = HO or NC ) and a leaving group, LG H ...
Organic Chemistry Introduction
... Aromatic (May be Anti-aromatic) • Planar, cyclic molecules with 4 n electrons are much less stable than expected (anti-aromatic) ...
... Aromatic (May be Anti-aromatic) • Planar, cyclic molecules with 4 n electrons are much less stable than expected (anti-aromatic) ...
Conjugate addition_Clayden
... But what about the reactions on the right? Both products A and B have kept their carbonyl group (IR peak at 1710 cm–1) but have lost the C=C. Yet A, at least, is definitely an addition product because it contains a C≡N peak at 2200 cm–1. Well, the identities of A and B are revealed here: they are th ...
... But what about the reactions on the right? Both products A and B have kept their carbonyl group (IR peak at 1710 cm–1) but have lost the C=C. Yet A, at least, is definitely an addition product because it contains a C≡N peak at 2200 cm–1. Well, the identities of A and B are revealed here: they are th ...
Enantioselective Organocatalytic Aminomethylation of Aldehydes: A
... formaldehyde derivatives, such as A, that can generate a methylene iminium species in situ.6 We examined L-proline and chiral pyrrolidines as catalysts for nucleophilic activation of aldehyde reactants. The Mannich reaction products, R-substituted β-amino aldehydes, were immediately reduced to the c ...
... formaldehyde derivatives, such as A, that can generate a methylene iminium species in situ.6 We examined L-proline and chiral pyrrolidines as catalysts for nucleophilic activation of aldehyde reactants. The Mannich reaction products, R-substituted β-amino aldehydes, were immediately reduced to the c ...