Factors that affect the rate of reactions
... Thermochemical equations: (“Heat Term” is in the equation. NO “H” shown beside the equation!) - “heat term” shown on left side of arrow - endothermic (“it uses up heat like a reactant”) eg. ...
... Thermochemical equations: (“Heat Term” is in the equation. NO “H” shown beside the equation!) - “heat term” shown on left side of arrow - endothermic (“it uses up heat like a reactant”) eg. ...
Reaction Kinetics
... • A catalyst lowers the activation energy required for the reaction to occur. By lowering the activation energy, the chemical reaction can occur much more quickly. ...
... • A catalyst lowers the activation energy required for the reaction to occur. By lowering the activation energy, the chemical reaction can occur much more quickly. ...
Chemistry - NTU.edu - Nanyang Technological University
... Chemical equilibria: reversible reactions; dynamic equilibrium (a) Factors affecting chemical equilibria (b) Equilibrium constants (c) The Haber process ...
... Chemical equilibria: reversible reactions; dynamic equilibrium (a) Factors affecting chemical equilibria (b) Equilibrium constants (c) The Haber process ...
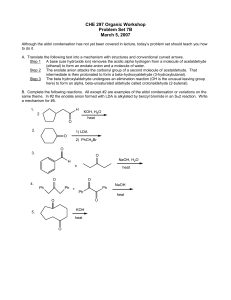
CHE 297 Organic Workshop
... (ethanal) to form an enolate anion and a molecule of water. Step 2 The enolate anion attacks the carbonyl group of a second molecule of acetaldehyde. That intermediate is then protonated to form a beta-hydroxyaldehyde (3-hydroxybutanal). Step 3 The beta hydroxylaldehyde undergoes an elimination reac ...
... (ethanal) to form an enolate anion and a molecule of water. Step 2 The enolate anion attacks the carbonyl group of a second molecule of acetaldehyde. That intermediate is then protonated to form a beta-hydroxyaldehyde (3-hydroxybutanal). Step 3 The beta hydroxylaldehyde undergoes an elimination reac ...
Reaction Rate review questions
... Show that the sum of the two steps in the reaction mechanism is the same as the overall equation for the reaction. What is the rate-determining step? Explain. First step is the slowest so it is the rate determining step. Identify any intermediates or catalysts. F is an intermediate, no catalyst. Pre ...
... Show that the sum of the two steps in the reaction mechanism is the same as the overall equation for the reaction. What is the rate-determining step? Explain. First step is the slowest so it is the rate determining step. Identify any intermediates or catalysts. F is an intermediate, no catalyst. Pre ...
Spontaniety
... -Entropy, like enthalpy is a state property so that ΔS = Sfinal - Sinitial ◦ unit for S = J/mol K ...
... -Entropy, like enthalpy is a state property so that ΔS = Sfinal - Sinitial ◦ unit for S = J/mol K ...
Le Chatelier`s Principle Quiz Answer Key
... 9. What factors alter the equilibrium position in chemical reactions? 10. Describe Le Chatelier's principle. 11. If more reactant is added to an equilibrium system, what happens to Keq (assume the temperature is not changed)? 12. How is changing the concentration of a reactant in a reaction related ...
... 9. What factors alter the equilibrium position in chemical reactions? 10. Describe Le Chatelier's principle. 11. If more reactant is added to an equilibrium system, what happens to Keq (assume the temperature is not changed)? 12. How is changing the concentration of a reactant in a reaction related ...
Sample
... Samples questions for advanced chemistry G12 This review is meant to help you sort through the different types of questions that meet the standards ...
... Samples questions for advanced chemistry G12 This review is meant to help you sort through the different types of questions that meet the standards ...
Biochemistry I (CHE 418 / 5418)
... • 3. Balance equation so that the same number of atoms of each element are on the left as on the right. – Add coefficients in FRONT of chemical formula; NEVER change subscripts on formulas – Start by balancing an element that appears in only one species on each ...
... • 3. Balance equation so that the same number of atoms of each element are on the left as on the right. – Add coefficients in FRONT of chemical formula; NEVER change subscripts on formulas – Start by balancing an element that appears in only one species on each ...