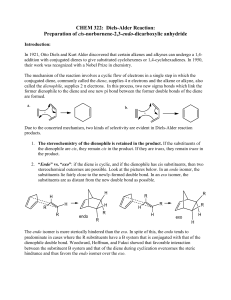
Cheat Sheet for Chemical Equilibrium
... • Given: Initial Concentration of Reactants only‐ Products will be zero. Determine the change by subtracting “x” from reactants and adding “x” to products. • Given: Initial Concentrations of Products only‐ Reactants will be zero. Determine the change by subtracting “x” from the products and addin ...
... • Given: Initial Concentration of Reactants only‐ Products will be zero. Determine the change by subtracting “x” from reactants and adding “x” to products. • Given: Initial Concentrations of Products only‐ Reactants will be zero. Determine the change by subtracting “x” from the products and addin ...
Template for calculating the ΔH° in a multiple step chemical reaction
... total ΔH° =_________ Write the two steps in the proper order... How to do it... To make Al2O3, you need the O3 from Fe2O3. To do that, you must first carry out the process of breaking up the Fe2O3, which has its own enthalpy of formation. However we are not forming Fe2O3. We are decomposing it. So, ...
... total ΔH° =_________ Write the two steps in the proper order... How to do it... To make Al2O3, you need the O3 from Fe2O3. To do that, you must first carry out the process of breaking up the Fe2O3, which has its own enthalpy of formation. However we are not forming Fe2O3. We are decomposing it. So, ...
Pb2+ +2I- → PbI2 (s)
... If given Hf or Sf or Gf, what do you do? Sum of products – sum of reactants (multiply by # of moles) ...
... If given Hf or Sf or Gf, what do you do? Sum of products – sum of reactants (multiply by # of moles) ...
Chapter 7 Chemical Reactions
... There are millions of compounds that will produce endless chemical reactions, therefore not all chemical reactions can be carried out in the laboratory A system is used to classify chemical reactions, which allows chemist to recognize patterns and predict the products of reactions One of these ...
... There are millions of compounds that will produce endless chemical reactions, therefore not all chemical reactions can be carried out in the laboratory A system is used to classify chemical reactions, which allows chemist to recognize patterns and predict the products of reactions One of these ...
Document
... Silicon carbide (SiC) is made from sand (silicon dioxide, SiO2) and carbon at high T. CO is also formed. If 100.0 kg of sand are reacted and 55.0 kg SiC are formed, what is the percent yield? SiO2(s) + 3 C(s) → SiC(s) + 2 CO(g) Convert kg SiO2 to moles: ...
... Silicon carbide (SiC) is made from sand (silicon dioxide, SiO2) and carbon at high T. CO is also formed. If 100.0 kg of sand are reacted and 55.0 kg SiC are formed, what is the percent yield? SiO2(s) + 3 C(s) → SiC(s) + 2 CO(g) Convert kg SiO2 to moles: ...
Thermochemistry
... Net ionic equation: Ba2+(aq) + SO42-(aq) ---> BaSO4(s) Mass of solution: 2L=2000ml=2000g Temperature increase: 28.1-25.0= 3.1°C ...
... Net ionic equation: Ba2+(aq) + SO42-(aq) ---> BaSO4(s) Mass of solution: 2L=2000ml=2000g Temperature increase: 28.1-25.0= 3.1°C ...
Review 3 - Bonham Chemistry
... 21. Industrially, we often need ethanoic acid. The starting material for this product is usually ethane. Show below a series of reactions that would transform ethane to ethanoic acid. ...
... 21. Industrially, we often need ethanoic acid. The starting material for this product is usually ethane. Show below a series of reactions that would transform ethane to ethanoic acid. ...
Screening - Entrance
... (ii) Write the zwitterionic structure. (iii) Write the structures of the amino acids obtained from the hydrolysis of aspartame. (iv) Which of the two amino acids is more hydrophobic? 8. An alkene (A) C16H16 on ozonolysis gives only one product (B) C8H8O. Compound (B) on reaction with NaOH/I2 yields ...
... (ii) Write the zwitterionic structure. (iii) Write the structures of the amino acids obtained from the hydrolysis of aspartame. (iv) Which of the two amino acids is more hydrophobic? 8. An alkene (A) C16H16 on ozonolysis gives only one product (B) C8H8O. Compound (B) on reaction with NaOH/I2 yields ...
South Pasadena • AP Chemistry
... 5. What three things must be taken into account when determining if a reaction has enough energy to overcome the activation energy (Ea)? 6. What does a catalyst do to a reaction? Explain. Something that is added to a reaction to speed up the reaction – it lowers the activation energy (energy necessa ...
... 5. What three things must be taken into account when determining if a reaction has enough energy to overcome the activation energy (Ea)? 6. What does a catalyst do to a reaction? Explain. Something that is added to a reaction to speed up the reaction – it lowers the activation energy (energy necessa ...
Chap Thirteen: Alcohols
... o 1° via SN2 Mechanism with ZnCl2 as catalyst ii. rxn w Thionyl chloride SOCl2 to form alkyl chlorides o SN2 and SN1 Mechanisms compete/ no rearrangement/ inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mech ...
... o 1° via SN2 Mechanism with ZnCl2 as catalyst ii. rxn w Thionyl chloride SOCl2 to form alkyl chlorides o SN2 and SN1 Mechanisms compete/ no rearrangement/ inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mech ...
Kazzie`s Guide to Orgo 2
... Guide to Orgo 2 General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 21 ...
... Guide to Orgo 2 General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 21 ...