Nugget
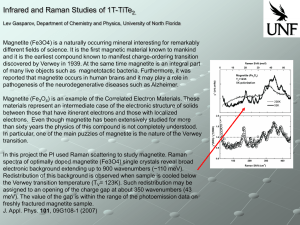
... discovered by Verwey in 1939. At the same time magnetite is an integral part of many live objects such as magnetotactic bacteria. Furthermore, it was reported that magnetite occurs in human brains and it may play a role in pathogenesis of the neurodegenerative diseases such as Alzheimer. Magnetite ( ...
... discovered by Verwey in 1939. At the same time magnetite is an integral part of many live objects such as magnetotactic bacteria. Furthermore, it was reported that magnetite occurs in human brains and it may play a role in pathogenesis of the neurodegenerative diseases such as Alzheimer. Magnetite ( ...
Hebden V.2 – Oxidation Numbers
... Oxidation numbers: charge an atom would have if the species containing the atom where made up of ions The sum of all positive charges and negative charges must equal the overall charge on the species Step 1: write the formula for the molecule Step 2: write the known oxidation numbers or charges belo ...
... Oxidation numbers: charge an atom would have if the species containing the atom where made up of ions The sum of all positive charges and negative charges must equal the overall charge on the species Step 1: write the formula for the molecule Step 2: write the known oxidation numbers or charges belo ...
AP CHEMISTRY COURSE SYLLABUS
... perform laboratory activities on any given day. THEMES: This course is centered on the 4 major themes, Structure of Matter, States of Matter, Chemical Reactions, and Descriptive Chemistry, listed in the AP Chemistry description. Within each of these themes, there will be topics that are extensions o ...
... perform laboratory activities on any given day. THEMES: This course is centered on the 4 major themes, Structure of Matter, States of Matter, Chemical Reactions, and Descriptive Chemistry, listed in the AP Chemistry description. Within each of these themes, there will be topics that are extensions o ...
chemical reactions and energy changes
... Let us look at what happens when an acid and a base react. Take as an example the reaction between aqueous solutions of hydrogen chloride (hydrochloric acid) and sodium hydroxide . Writing the equation in terms of aqueous ions gives: ...
... Let us look at what happens when an acid and a base react. Take as an example the reaction between aqueous solutions of hydrogen chloride (hydrochloric acid) and sodium hydroxide . Writing the equation in terms of aqueous ions gives: ...
Inorganic Chemistry 412 / 512
... The inert pair effect. Pb forms weaker bonds than Sn, and the highest oxidation state is therefore less stable. ...
... The inert pair effect. Pb forms weaker bonds than Sn, and the highest oxidation state is therefore less stable. ...
File
... molecule averaged over similar compounds Calculation of the enthalpy changes from known bond enthalpy values and comparison of these to experimentally measured values Sketching and evaluation of potential energy profiles in determining whether reactants or products are more stable and if the reactio ...
... molecule averaged over similar compounds Calculation of the enthalpy changes from known bond enthalpy values and comparison of these to experimentally measured values Sketching and evaluation of potential energy profiles in determining whether reactants or products are more stable and if the reactio ...
Review for Final Exam - Short Answer and Problems
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate and ...
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate and ...
Chemistry 520 - Physical Chemistry
... counting 30%. The exams are tentatively scheduled for: 1st Midterm: Tuesday February 4,7-8:30 P.M. 2nd Midterm: Tuesday February 25, 7-8:30 P.M. Final Exam: Tuesday March 18, 11:30-1:18, 2004 Evans Lab (time set by the University) Homework: Weekly homework problems will count 20% of your final grade ...
... counting 30%. The exams are tentatively scheduled for: 1st Midterm: Tuesday February 4,7-8:30 P.M. 2nd Midterm: Tuesday February 25, 7-8:30 P.M. Final Exam: Tuesday March 18, 11:30-1:18, 2004 Evans Lab (time set by the University) Homework: Weekly homework problems will count 20% of your final grade ...
preliminary course outline facilitators course description
... solving quadratic equations, knowledge of logarithms and basic calculus will also be necessary. The course is structured around Chapters 13-18 of the course text in which we examine how and why chemical reactions occur. In Chapter 13 we examine how quickly chemical reactions occur, and how catalysts ...
... solving quadratic equations, knowledge of logarithms and basic calculus will also be necessary. The course is structured around Chapters 13-18 of the course text in which we examine how and why chemical reactions occur. In Chapter 13 we examine how quickly chemical reactions occur, and how catalysts ...
RTF
... 2. For systems involving gases, the equilibrium constant is often determined by using partial pressure instead of concentration. Given the following reaction at equilibrium at the partial pressures of the ...
... 2. For systems involving gases, the equilibrium constant is often determined by using partial pressure instead of concentration. Given the following reaction at equilibrium at the partial pressures of the ...
Chapter 7 - Chemical Reactions
... Use standard enthalpies of formation from Table C-13 (attached) to calculate ΔHreaction for each of these reactions. a. 2H2S(g) + 3O2(g) →2H2O(g) + 2SO2(g) CHAPTER 17 OBJECTIVES Calculate the molarity of a solution that contains 50.0 g of NaCl per 0.6 L of solution. How many moles of solute are pres ...
... Use standard enthalpies of formation from Table C-13 (attached) to calculate ΔHreaction for each of these reactions. a. 2H2S(g) + 3O2(g) →2H2O(g) + 2SO2(g) CHAPTER 17 OBJECTIVES Calculate the molarity of a solution that contains 50.0 g of NaCl per 0.6 L of solution. How many moles of solute are pres ...
PPT: Chemical Reactions Review
... Temperature at which reaction is carried out, in this case 0 oC ...
... Temperature at which reaction is carried out, in this case 0 oC ...
1P18 IR spectroscopic investigation on intermolecular proton
... performed at the PBE1PBE/6-31+G* level. ...
... performed at the PBE1PBE/6-31+G* level. ...