here
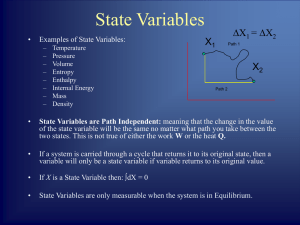
... • Every thermodynamic system in an equilibrium state possesses a state variable called the internal energy U whose change in a differential process is given by ...
... • Every thermodynamic system in an equilibrium state possesses a state variable called the internal energy U whose change in a differential process is given by ...
First law of thermodynamics
... The units for work and heat or for any other form of energy either are the same or are directly proportional. In SI units, the joule is used as the unit for both work and heat ad for any other energy unit. In English units, the basic unit for work is the foot pound force, and the basic unit for hea ...
... The units for work and heat or for any other form of energy either are the same or are directly proportional. In SI units, the joule is used as the unit for both work and heat ad for any other energy unit. In English units, the basic unit for work is the foot pound force, and the basic unit for hea ...
Quiz_MATH.rtf
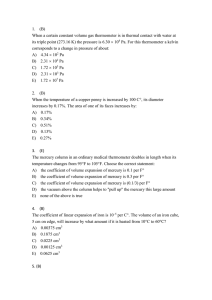
... D) decreases at high temperature, increases at low E) stays the same 13. (C) Two monatomic ideal gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular kinetic energy KA/KB is ...
... D) decreases at high temperature, increases at low E) stays the same 13. (C) Two monatomic ideal gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular kinetic energy KA/KB is ...
Summary presentation 10.2 File
... In order to distinguish between heat and work in thermodynamic processes Heat is defined as a process in which thermal energy is transferred due to a temperature difference. Work is defined as the process in which thermal energy is transferred by means that are independent of a temperature differenc ...
... In order to distinguish between heat and work in thermodynamic processes Heat is defined as a process in which thermal energy is transferred due to a temperature difference. Work is defined as the process in which thermal energy is transferred by means that are independent of a temperature differenc ...
Concepts for specific heat
... Thus the average energy is just twice as large as for the free particle, as the energy is evenly distributed between the kinetic and potential energy. (This only holds for quadratic potentials!) In thermal equilibrium each oscillation mode has the internal energy E = kB T ...
... Thus the average energy is just twice as large as for the free particle, as the energy is evenly distributed between the kinetic and potential energy. (This only holds for quadratic potentials!) In thermal equilibrium each oscillation mode has the internal energy E = kB T ...
lecture notes-growth kinetics-3-heat evolution
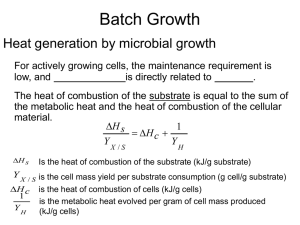
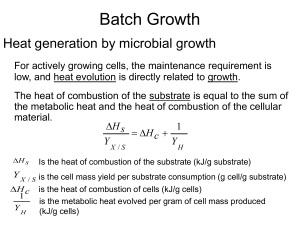
... For actively growing cells, the maintenance requirement is ...
... For actively growing cells, the maintenance requirement is ...
Chapter 6:
... c. The equivalence of heat and work. d. The equivalence of internal energy and work. Answer: A 4. The statement, the internal work of an isolated system is constant, is also known as what? a. The first law of thermodynamics. b. The law of conservation of energy. c. The law of conservation of mass. d ...
... c. The equivalence of heat and work. d. The equivalence of internal energy and work. Answer: A 4. The statement, the internal work of an isolated system is constant, is also known as what? a. The first law of thermodynamics. b. The law of conservation of energy. c. The law of conservation of mass. d ...
AP Physics - Thermodynamics
... U is the change in internal energy of the system, Q is the heat added to the system, and W is the work added to the system (or done on the system). Q is positive when it is added to the system and negative if it is taken out of the system. W is positive if it is added to the system and negative if ...
... U is the change in internal energy of the system, Q is the heat added to the system, and W is the work added to the system (or done on the system). Q is positive when it is added to the system and negative if it is taken out of the system. W is positive if it is added to the system and negative if ...
Thermodynamics
... determined that a more fundamental law had been neglected, apparently because it had seemed so obvious that it did not need to be stated explicitly. To form a complete set of rules, scientists decided this most fundamental law needed to be included. The problem, though, was that the first three laws ...
... determined that a more fundamental law had been neglected, apparently because it had seemed so obvious that it did not need to be stated explicitly. To form a complete set of rules, scientists decided this most fundamental law needed to be included. The problem, though, was that the first three laws ...
Chapter 9: Thermodynamic Processes and Thermochemistry
... KEav = (3/2) RT Random motion is often called thermal motion. Heat involves the transfer of energy between two objects due to a temperature difference between the two objects. When a hot body is brought into contact with a colder body, the two temperatures change until they become equal to one anoth ...
... KEav = (3/2) RT Random motion is often called thermal motion. Heat involves the transfer of energy between two objects due to a temperature difference between the two objects. When a hot body is brought into contact with a colder body, the two temperatures change until they become equal to one anoth ...
Thermodynamics: Heat and Work
... • If a gas expands rapidly its temperature, pressure, and internal energy decrease. • If this happens in a closed environment, no heat can be transferred to or from the environment, such a process is called an adiabatic process from a Greek word meaning impassible ...
... • If a gas expands rapidly its temperature, pressure, and internal energy decrease. • If this happens in a closed environment, no heat can be transferred to or from the environment, such a process is called an adiabatic process from a Greek word meaning impassible ...
Document
... At constant pressure, the change in enthalpy is equal to the heat The change of enthalpy is independent of path. Q: Does q or W depend on path? For the change involving solids and liquids, HU, but for gases, HU Q:explain why? ...
... At constant pressure, the change in enthalpy is equal to the heat The change of enthalpy is independent of path. Q: Does q or W depend on path? For the change involving solids and liquids, HU, but for gases, HU Q:explain why? ...
thermochem-prob-solns
... energy of the system. U is the change in internal energy of system. It is a state function. q- heat energy absorbed (q is positive) or given off (q is negative) by the system. w- work done by the system(w is negative) on its surroundings or done on the system(w is positive) by its surroundings. Ex ...
... energy of the system. U is the change in internal energy of system. It is a state function. q- heat energy absorbed (q is positive) or given off (q is negative) by the system. w- work done by the system(w is negative) on its surroundings or done on the system(w is positive) by its surroundings. Ex ...
Notes on the First Law of Thermodynamics Chemistry CHEM 213W
... heights in the surroundings. In doing so, the system (the spring and pan) move from state I to II. How much work is performed in each of the cases (a)−(c) (assume that there is a total 1cm elongation of the spring and ignore the mass of the pan and spring)? In order to reverse the process (i.e., the ...
... heights in the surroundings. In doing so, the system (the spring and pan) move from state I to II. How much work is performed in each of the cases (a)−(c) (assume that there is a total 1cm elongation of the spring and ignore the mass of the pan and spring)? In order to reverse the process (i.e., the ...
Thermodynamics - myersparkphysics
... Is there an IDEAL engine model? Our goal is to figure out just how efficient such a heat engine can be: what’s the most work we can possibly get for a given amount of fuel? The efficiency question was first posed—and solved—by Sadi Carnot in 1820, not long after steam engines had become efficient e ...
... Is there an IDEAL engine model? Our goal is to figure out just how efficient such a heat engine can be: what’s the most work we can possibly get for a given amount of fuel? The efficiency question was first posed—and solved—by Sadi Carnot in 1820, not long after steam engines had become efficient e ...
Thermodynamics
... Is there an IDEAL engine model? Our goal is to figure out just how efficient such a heat engine can be: what’s the most work we can possibly get for a given amount of fuel? The efficiency question was first posed—and solved—by Sadi Carnot in 1820, not long after steam engines had become efficient e ...
... Is there an IDEAL engine model? Our goal is to figure out just how efficient such a heat engine can be: what’s the most work we can possibly get for a given amount of fuel? The efficiency question was first posed—and solved—by Sadi Carnot in 1820, not long after steam engines had become efficient e ...
Lecture 2 - Richard Grotjahn
... from one form to another • Equations for some forms of energy: – Potential energy: PE = M*g*z g=acceleration of gravity, z=elevation, M=mass – Kinetic energy: KE = ½ * M * v2 v=velocity ...
... from one form to another • Equations for some forms of energy: – Potential energy: PE = M*g*z g=acceleration of gravity, z=elevation, M=mass – Kinetic energy: KE = ½ * M * v2 v=velocity ...
IA_Keep_the_Heat_Answers
... the lowest temperature is blue. What does this picture tell about the heat loss from the house? Describe in as much detail as possible. (This question is worth both points and is the First Tie Breaker) The area’s in this picture that are white or Red indicate the area’s where heat loss is the greate ...
... the lowest temperature is blue. What does this picture tell about the heat loss from the house? Describe in as much detail as possible. (This question is worth both points and is the First Tie Breaker) The area’s in this picture that are white or Red indicate the area’s where heat loss is the greate ...
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.