User Guide - OJEE 2015
... Effect, laws of transverse vibration of string (Statement only). Optics: Reflection and refraction at curved surfaces. Spherical mirror and thin lens formula and refraction through prism. Total internal reflection, Dispersion, Huygens principle (statement only), Young’s double slit experiment. Elec ...
... Effect, laws of transverse vibration of string (Statement only). Optics: Reflection and refraction at curved surfaces. Spherical mirror and thin lens formula and refraction through prism. Total internal reflection, Dispersion, Huygens principle (statement only), Young’s double slit experiment. Elec ...
BW1 - wlhs.wlwv.k12.or.us
... Solve: The thermal energy created in the tires and the road may be determined by: Eth WMark FMark d cos0 (110 N)(150 m)cos0 470 J Assess: All the work Mark does in pushing the car, becomes thermal energy of the tires and road. Since Mark is pushing in the direction the car is moving, the ...
... Solve: The thermal energy created in the tires and the road may be determined by: Eth WMark FMark d cos0 (110 N)(150 m)cos0 470 J Assess: All the work Mark does in pushing the car, becomes thermal energy of the tires and road. Since Mark is pushing in the direction the car is moving, the ...
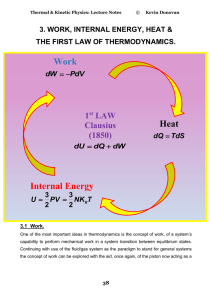
2. Thermodynamic Processes and Quantities Defined
... Since PV = nRT, that means it returns to the same temperature as well. U = 0, and the first law reduces to ___________. ...
... Since PV = nRT, that means it returns to the same temperature as well. U = 0, and the first law reduces to ___________. ...
Entropy and Free Energy
... An example is the polymerization of amino acids to form protenoids: a Amino acid A + b Amino acid B c polymer + d H2O La Chateliers principle indicates that this equilibrium would lie far to the left and entropy requirements will grow rapidly with molecular weight. Some small protenoids have been ...
... An example is the polymerization of amino acids to form protenoids: a Amino acid A + b Amino acid B c polymer + d H2O La Chateliers principle indicates that this equilibrium would lie far to the left and entropy requirements will grow rapidly with molecular weight. Some small protenoids have been ...
Ex. 36 PowerPoint
... When an object is decelerated, the kinetic energy it has must be dissipated (reduced to zero). When stopping a car, work is done by the brakes (friction) to dissipate the energy. The EK of the car is transformed into heat and sound. The fact that the kinetic energy of an object is proportional to th ...
... When an object is decelerated, the kinetic energy it has must be dissipated (reduced to zero). When stopping a car, work is done by the brakes (friction) to dissipate the energy. The EK of the car is transformed into heat and sound. The fact that the kinetic energy of an object is proportional to th ...
Provedení, principy činnosti a základy výpočtu pro výměníky tepla
... dTw=-dQwm/(mwcpw) and the temperature Tm increases by dTm=dQwm/(mmcpm), however the sum of inner energies U=ummm+uwmw remains, therefore dU=0. Volume V is constant and dV=0. Therefore the entropy increase of the whole system dS should be zero (dS=0) TdS dU pdV 0 ...
... dTw=-dQwm/(mwcpw) and the temperature Tm increases by dTm=dQwm/(mmcpm), however the sum of inner energies U=ummm+uwmw remains, therefore dU=0. Volume V is constant and dV=0. Therefore the entropy increase of the whole system dS should be zero (dS=0) TdS dU pdV 0 ...
First, there are several issues regarding this course need to be
... (The calculated result is slightly larger than the experimental value). Quite often, we do not have to go through the above process in order to know the standard Gibbs energy of formation or standard reaction Gibbs energy, which could be employed for further calculations such as calculating equilibr ...
... (The calculated result is slightly larger than the experimental value). Quite often, we do not have to go through the above process in order to know the standard Gibbs energy of formation or standard reaction Gibbs energy, which could be employed for further calculations such as calculating equilibr ...
ATOMIC BATTERY BASED ON
... occur in air. After several minutes, the contours of the stratified structure are disturbed because of a continuous loss of particles on the walls. Both in neon and air, the dust component is in motion which covers the entire volume and takes a stable form several seconds after the injection of the ...
... occur in air. After several minutes, the contours of the stratified structure are disturbed because of a continuous loss of particles on the walls. Both in neon and air, the dust component is in motion which covers the entire volume and takes a stable form several seconds after the injection of the ...
Infrared Thermometry
... intensity) and output (temperature reading). The output is typically routed into a control system and used as feedback to adjust the process in ...
... intensity) and output (temperature reading). The output is typically routed into a control system and used as feedback to adjust the process in ...
g - WordPress.com
... of moles of HCl and NaOH involved, and the density and specific heat of the solution. Plan The total heat produced can be calculated using Equation 5.23. The number of moles of HCl consumed in the reaction must be calculated from the volume and molarity of this substance, and this amount is then use ...
... of moles of HCl and NaOH involved, and the density and specific heat of the solution. Plan The total heat produced can be calculated using Equation 5.23. The number of moles of HCl consumed in the reaction must be calculated from the volume and molarity of this substance, and this amount is then use ...
down
... complicated trend for lighter molecules) Bond length : increased as bond energy and force constant decreases These data can be qualitatively understood using MO theory ...
... complicated trend for lighter molecules) Bond length : increased as bond energy and force constant decreases These data can be qualitatively understood using MO theory ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.