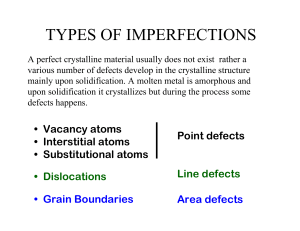
section_3.2
... To understand and illustrate the Law of constant composition To learn how a formula describes a compound’s composition ...
... To understand and illustrate the Law of constant composition To learn how a formula describes a compound’s composition ...
Intermolecular Forces
... Ara.CH4, or Arm.xyclopropane, there is, in addition to the dispersion energy terms in R4, R-’, R-lo, ..., an orientation-dependent contribution that varies as K’. It could be significant for coupling the translation and rotation in gases and liquids and for the lattice energy of solids.* London illu ...
... Ara.CH4, or Arm.xyclopropane, there is, in addition to the dispersion energy terms in R4, R-’, R-lo, ..., an orientation-dependent contribution that varies as K’. It could be significant for coupling the translation and rotation in gases and liquids and for the lattice energy of solids.* London illu ...
Chapter 5 Thermochemistry
... SAMPLE EXERCISE 5.2 Relating heat and work to changes of internal energy Two gases, A(g) and B(g), are confined in a cylinder-and-piston arrangement like that in Figure 5.3. Substances A and B react to form a solid product: As the reactions occurs, the system loses 1150 J of heat to the surrounding. ...
... SAMPLE EXERCISE 5.2 Relating heat and work to changes of internal energy Two gases, A(g) and B(g), are confined in a cylinder-and-piston arrangement like that in Figure 5.3. Substances A and B react to form a solid product: As the reactions occurs, the system loses 1150 J of heat to the surrounding. ...
Static and dynamic thermal characterisation of a hollow brick wall
... r (kg/m ) k (W/m K) c (J/kg K) ...
... r (kg/m ) k (W/m K) c (J/kg K) ...
Elements, Compounds and Mixtures
... 8. A chocolate-chip cookie with more chips in one part of the cookie than another can be used to demonstrate a heterogeneous mixture. Name two other materials that can be classified as heterogeneous mixtures. Explain your reasoning. ...
... 8. A chocolate-chip cookie with more chips in one part of the cookie than another can be used to demonstrate a heterogeneous mixture. Name two other materials that can be classified as heterogeneous mixtures. Explain your reasoning. ...
Chapter 5 Thermochemistry
... SAMPLE EXERCISE 5.2 Relating heat and work to changes of internal energy Two gases, A(g) and B(g), are confined in a cylinder-and-piston arrangement like that in Figure 5.3. Substances A and B react to form a solid product: As the reactions occurs, the system loses 1150 J of heat to the surrounding. ...
... SAMPLE EXERCISE 5.2 Relating heat and work to changes of internal energy Two gases, A(g) and B(g), are confined in a cylinder-and-piston arrangement like that in Figure 5.3. Substances A and B react to form a solid product: As the reactions occurs, the system loses 1150 J of heat to the surrounding. ...
NCEA Level 3 Physics (91524) 2016 Assessment Schedule
... Correct damped shape for 3 complete cycles and constant period and at least one value on axes (A0 = 20 cm, and T = 1.57 s). OR States assumptions of zero damping and the graph shows this ...
... Correct damped shape for 3 complete cycles and constant period and at least one value on axes (A0 = 20 cm, and T = 1.57 s). OR States assumptions of zero damping and the graph shows this ...
Year 10 revision checklist69.83 KB
... The use of high energy ionising radiation can be dangerous, and precautions need to be taken to monitor and minimise the levels of radiation that people who work with it are exposed to. Sound and Ultrasound: Sound waves are longitudinal waves and cause vibrations in a medium, which are detected as s ...
... The use of high energy ionising radiation can be dangerous, and precautions need to be taken to monitor and minimise the levels of radiation that people who work with it are exposed to. Sound and Ultrasound: Sound waves are longitudinal waves and cause vibrations in a medium, which are detected as s ...
CHAP4
... following (also assuming isobaric conditions): a) the change in internal energy b) the change in enthalpy c) the change in entropy 2. Calculate the change in entropy of 2 g of ice initially at -10 C which is converted to steam at 100 C due to heating. [ans: 17.3 J K-1] 3. A 200 g sample of dry air ...
... following (also assuming isobaric conditions): a) the change in internal energy b) the change in enthalpy c) the change in entropy 2. Calculate the change in entropy of 2 g of ice initially at -10 C which is converted to steam at 100 C due to heating. [ans: 17.3 J K-1] 3. A 200 g sample of dry air ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.