Chapter 15
... A special kind of periodic motion occurs in mechanical systems when the force acting on the object is proportional to the position of the object relative to some equilibrium position. If the force is always directed toward the equilibrium position, the motion is called simple harmonic motion. ...
... A special kind of periodic motion occurs in mechanical systems when the force acting on the object is proportional to the position of the object relative to some equilibrium position. If the force is always directed toward the equilibrium position, the motion is called simple harmonic motion. ...
CHAPTER 6
... potential. Starting from this definition, after some manipulations, it is possible to derive the well-known Nerst-Plank equation: ...
... potential. Starting from this definition, after some manipulations, it is possible to derive the well-known Nerst-Plank equation: ...
Chapter 3. Thermodynamics and Electrochemical Kinetics
... the disorder in a system. A process that does not generate entropy is called a reversible process if it can be performed and then returned to its initial state (reversed) without leaving any traces on the surroundings. Therefore, in a reversible process, by the First Law, no net exchange of heat or ...
... the disorder in a system. A process that does not generate entropy is called a reversible process if it can be performed and then returned to its initial state (reversed) without leaving any traces on the surroundings. Therefore, in a reversible process, by the First Law, no net exchange of heat or ...
Physical Limits of Computing
... The few remaining serious puzzles in physics, such as the origin of mass, the disparity between the strengths of the fundamental forces, and the unification of general relativity and quantum mechanics are all of a rather abstract and aesthetic flavor. Their eventual resolution (whatever form it take ...
... The few remaining serious puzzles in physics, such as the origin of mass, the disparity between the strengths of the fundamental forces, and the unification of general relativity and quantum mechanics are all of a rather abstract and aesthetic flavor. Their eventual resolution (whatever form it take ...
Basics of Magnetism - Raja Ramanna Centre for Advanced
... Details: If E is towards a fixed nucleus, classically all LX, LY, LZ are constants (fixed plane for a central force). In QM LZ & L2 are constants of motion but in a non-central field (as in a crystal) the orbital plane is not fixed & the components of L are not constants & may be even zero on the av ...
... Details: If E is towards a fixed nucleus, classically all LX, LY, LZ are constants (fixed plane for a central force). In QM LZ & L2 are constants of motion but in a non-central field (as in a crystal) the orbital plane is not fixed & the components of L are not constants & may be even zero on the av ...
Law conservation of energy worksheet
... The Law of Conservation of Energy states that energy cannot be created or. Learn about the Law of Conservation of Energy and how energy changes from potential energy to kinetic energy. This lesson provides a problem that reviews the. Grade 11 Physics Cumulative. Gravity, Pendulums, and the Conservat ...
... The Law of Conservation of Energy states that energy cannot be created or. Learn about the Law of Conservation of Energy and how energy changes from potential energy to kinetic energy. This lesson provides a problem that reviews the. Grade 11 Physics Cumulative. Gravity, Pendulums, and the Conservat ...
Document
... freely (against zero external pressure). For the three processes calculate final pressure Pf , q, w, ΔU, and ΔH. Ans: (a) (b) (c) ...
... freely (against zero external pressure). For the three processes calculate final pressure Pf , q, w, ΔU, and ΔH. Ans: (a) (b) (c) ...
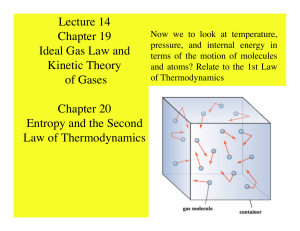
Lecture 14 Chapter 19 Ideal Gas Law and Kinetic Theory of Gases
... Note that units are Joule per kelvin and the sign is the same as Q since T > 0 • However, the above formula can only be used to calculate the entropy change if the process is reversible.. • To find the entropy for an irreversible process and since state functions only depend on the end points, the t ...
... Note that units are Joule per kelvin and the sign is the same as Q since T > 0 • However, the above formula can only be used to calculate the entropy change if the process is reversible.. • To find the entropy for an irreversible process and since state functions only depend on the end points, the t ...
sy16_oct26_f11a
... How much will the spring compress (i.e. x = xf - xi) to bring the box to a stop (i.e., v = 0 ) if the object is moving initially at a constant velocity (vo) on frictionless surface as shown below ? x Wbox F ( x ) dx to vo x x m ...
... How much will the spring compress (i.e. x = xf - xi) to bring the box to a stop (i.e., v = 0 ) if the object is moving initially at a constant velocity (vo) on frictionless surface as shown below ? x Wbox F ( x ) dx to vo x x m ...
S3 Numeracy Booklets – Atomic Structure
... What number is equal to the atomic mass of carbon + the atomic number of magnesium? Ans: atomic mass of carbon is 12; atomic number of magnesium is 12, so 12 + 12 = 24. Extra marks will be awarded for ingenuity and complexity. ...
... What number is equal to the atomic mass of carbon + the atomic number of magnesium? Ans: atomic mass of carbon is 12; atomic number of magnesium is 12, so 12 + 12 = 24. Extra marks will be awarded for ingenuity and complexity. ...
lec01
... Statistical Mechanics In the late 19th and early 20th century, with the discovery of atoms, thermodynamics was treated in terms of atoms and molecules, on a statistical basis, and the subject of statistical mechanics came about. ...
... Statistical Mechanics In the late 19th and early 20th century, with the discovery of atoms, thermodynamics was treated in terms of atoms and molecules, on a statistical basis, and the subject of statistical mechanics came about. ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.