Chapter 5 Thermochemistry - Byron Senior High School
... • An older, non-SI unit is still in widespread use: The calorie (cal). 1 cal = 4.184 J Thermochemistry ...
... • An older, non-SI unit is still in widespread use: The calorie (cal). 1 cal = 4.184 J Thermochemistry ...
Potential of Quasicrystals and Quasicrystal Approximants for New
... These thermal conductivity values resemble those of amorphous glasses which typically have k& WlmeK.2’ ...
... These thermal conductivity values resemble those of amorphous glasses which typically have k& WlmeK.2’ ...
potential energy curves, motion, turning points
... world of atoms and molecules the concept of force does not exist and the potential energy function replaces it as the prime quantity of interest. In this module we will work with you on understanding how one uses the potential energy function to deduce motion in classical physics. That means we will ...
... world of atoms and molecules the concept of force does not exist and the potential energy function replaces it as the prime quantity of interest. In this module we will work with you on understanding how one uses the potential energy function to deduce motion in classical physics. That means we will ...
Chemical Bond Activation Observed with an X
... that are spread in energy over several electronvolts above and below the Fermi level with a constantly low density of states are derived from the interaction of the oxygen 2p level with the broad Ru 5s band. On the other hand, the interaction with welllocalized Ru 4d states yields two prominent feat ...
... that are spread in energy over several electronvolts above and below the Fermi level with a constantly low density of states are derived from the interaction of the oxygen 2p level with the broad Ru 5s band. On the other hand, the interaction with welllocalized Ru 4d states yields two prominent feat ...
PDF
... Kinematic viscosity of the fluid, Velocity of flow Density of material Viscosity of fluid Thermal diffusivity. Specific heat ...
... Kinematic viscosity of the fluid, Velocity of flow Density of material Viscosity of fluid Thermal diffusivity. Specific heat ...
Powerpoint
... Kinetic energy is always K = ½ mv2 = ½ m(A)2 sin2(t+) Potential energy of a spring is, U = ½ k x2 = ½ k A2 cos2(t + ) And 2 = k / m or k = m 2 U = ½ m 2 A2 cos2(t + ) Physics 207: Lecture 19, Pg 22 ...
... Kinetic energy is always K = ½ mv2 = ½ m(A)2 sin2(t+) Potential energy of a spring is, U = ½ k x2 = ½ k A2 cos2(t + ) And 2 = k / m or k = m 2 U = ½ m 2 A2 cos2(t + ) Physics 207: Lecture 19, Pg 22 ...
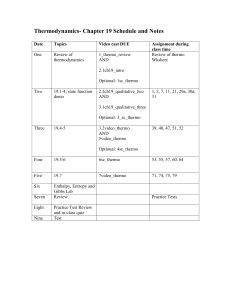
Structure of Thrmodynamics
... A finite rate of change (or a finite rate of a rate of change) cannot be stopped by means of infinitesimal alteration in the circumstances. (J.W. Gibbs, Collected Works, Yale University. Press, Vol. 1 p.56,1948) ...
... A finite rate of change (or a finite rate of a rate of change) cannot be stopped by means of infinitesimal alteration in the circumstances. (J.W. Gibbs, Collected Works, Yale University. Press, Vol. 1 p.56,1948) ...
Ionic Solids
... • Alloys are materials that contain more than one element and have the characteristic properties of metals. • It is used to change the properties of certain metals. ...
... • Alloys are materials that contain more than one element and have the characteristic properties of metals. • It is used to change the properties of certain metals. ...
University Physics AI No. 12 The Second Law of Thermodynamics
... (c)Calculate the work done by the gas during each path of the cycle and the total work done by the gas. And the heat transfer to the gas during each path of the cycle and the total heat transfer to the gas over the cycle. (d) Find the efficiency of the cycle. (e) Calculate the maximum efficiency tha ...
... (c)Calculate the work done by the gas during each path of the cycle and the total work done by the gas. And the heat transfer to the gas during each path of the cycle and the total heat transfer to the gas over the cycle. (d) Find the efficiency of the cycle. (e) Calculate the maximum efficiency tha ...
Salt Solutions Ionic Bonding
... There is an equilibrium between solid sodium chloride and the solvated ions Na+(aq) and Cl-(aq) when NaCl is added to water. Like all equilibria, an equilibrium constant is equal to the ratio of the concentrations of products to the concentrations of reactants. The concentration of any solid is def ...
... There is an equilibrium between solid sodium chloride and the solvated ions Na+(aq) and Cl-(aq) when NaCl is added to water. Like all equilibria, an equilibrium constant is equal to the ratio of the concentrations of products to the concentrations of reactants. The concentration of any solid is def ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.