CHAPTER Work and Energy
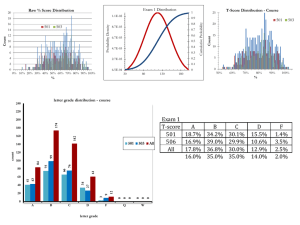
... 46 ·· A 700-kg car accelerates from rest under constant power at t = 0. At t = 9 s it is 117.7 m from its starting point and its acceleration is then 1.09 m/s2. Find the power expended by the car’s engine, neglecting frictional losses. From Example 6-11, P = 9mx2/8t3 P = [9 × 700 × (117.7) 2/8 × 93] ...
... 46 ·· A 700-kg car accelerates from rest under constant power at t = 0. At t = 9 s it is 117.7 m from its starting point and its acceleration is then 1.09 m/s2. Find the power expended by the car’s engine, neglecting frictional losses. From Example 6-11, P = 9mx2/8t3 P = [9 × 700 × (117.7) 2/8 × 93] ...
BONDING AND GEOMETRY
... Very strong type of dipole force Only occurs when hydrogen is covalently bonded to a highly electronegative atom Always involves hydrogen Example: HF, H2O, NH3 ...
... Very strong type of dipole force Only occurs when hydrogen is covalently bonded to a highly electronegative atom Always involves hydrogen Example: HF, H2O, NH3 ...
selection and evaluation of materials for thermoelectric applications
... It is clear that in order to obtain a high figure of merit, a large Seebeck coefficient and high electrical conductivity are required, as well as a low thermal conductivity. Although it is essentially true that the search for better thermoelectric materials amounts to maximizing ZT, there are nevert ...
... It is clear that in order to obtain a high figure of merit, a large Seebeck coefficient and high electrical conductivity are required, as well as a low thermal conductivity. Although it is essentially true that the search for better thermoelectric materials amounts to maximizing ZT, there are nevert ...
The upper temperature threshold of eggs is 40°C
... the temperature reached on the surface of the material is less then that required by conventional heating in order to attempt the same effect inside the body; the heating is more uniform for large bodies too; it could be possible to operate selectively by focussing microwave beam or by exploit ...
... the temperature reached on the surface of the material is less then that required by conventional heating in order to attempt the same effect inside the body; the heating is more uniform for large bodies too; it could be possible to operate selectively by focussing microwave beam or by exploit ...
Oxide Thermoelectric Materials for Heat-to
... While the CuO2 layers in the superconducting cuprates consist of planar square lattices of Cu-O, the CoO2 layers in the layered cobalt oxides consist of planar triangle lattices of Co-Co, suggesting a magnetic frustration in the spin configuration of conduction electrons. In NaCo2O4, CoO2 layers and ...
... While the CuO2 layers in the superconducting cuprates consist of planar square lattices of Cu-O, the CoO2 layers in the layered cobalt oxides consist of planar triangle lattices of Co-Co, suggesting a magnetic frustration in the spin configuration of conduction electrons. In NaCo2O4, CoO2 layers and ...
The First and Second Laws of Thermodynamics
... Energy is a conserved property, and no process is known to have taken place in violation of the first law of thermodynamics. Therefore, it is reasonable to conclude that a process must satisfy the first law to occur. However, as explained here, satisfying the first law alone does not ensure that the ...
... Energy is a conserved property, and no process is known to have taken place in violation of the first law of thermodynamics. Therefore, it is reasonable to conclude that a process must satisfy the first law to occur. However, as explained here, satisfying the first law alone does not ensure that the ...
Chapter 8: POTENTIAL ENERGY AND CONSERVATION OF ENERGY
... A. the potential energy changed from the beginning to the end of the trip B. mechanical energy was increased by nonconservative forces C. mechanical energy was decreased by nonconservative forces D. mechanical energy was increased by conservative forces E. mechanical energy was decreased by conserva ...
... A. the potential energy changed from the beginning to the end of the trip B. mechanical energy was increased by nonconservative forces C. mechanical energy was decreased by nonconservative forces D. mechanical energy was increased by conservative forces E. mechanical energy was decreased by conserva ...
Review IV
... Types of Intermolecular Forces: Dispersion, Dipole-Dipole, and Hydrogen Bonding A. Dispersion force, also known as London forces 1. Weakest intermolecular force due to Instantaneous dipole 2. Involved in every molecule/atom intermolecular interaction 3. Increases with molar mass 4. Only operative f ...
... Types of Intermolecular Forces: Dispersion, Dipole-Dipole, and Hydrogen Bonding A. Dispersion force, also known as London forces 1. Weakest intermolecular force due to Instantaneous dipole 2. Involved in every molecule/atom intermolecular interaction 3. Increases with molar mass 4. Only operative f ...
ECOLE POLYTECHNIQUE Mathematical Analysis of a Saint
... In the remaining part of the paper we derive the Saint-Venant equations with an energy equation taking into account the temperature dependence of transport coefficients. These equations are derived from an asymptotic study of a three dimensional incompressible model of a thin viscous sheet over a f ...
... In the remaining part of the paper we derive the Saint-Venant equations with an energy equation taking into account the temperature dependence of transport coefficients. These equations are derived from an asymptotic study of a three dimensional incompressible model of a thin viscous sheet over a f ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.