Advanced Physical Chemistry Professor Angelo R. Rossi http
... The sequence of situations the system goes through in passing from the initial state to the final state is called the path taken by the system. Because the intensive variables often have no values during a process, it is usually not possible to exactly specify the path a process takes in terms of th ...
... The sequence of situations the system goes through in passing from the initial state to the final state is called the path taken by the system. Because the intensive variables often have no values during a process, it is usually not possible to exactly specify the path a process takes in terms of th ...
Time-resolved nonlinear optical spectroscopy
... be conditioned by the relaxation of the excited electrons. The nonlinear symmetric perturbation of the tensor extends over a longer time span, and experiences a drastic change of its spectral dependence within the first 1.5 ps, the analysis of which led us to attribute its decay to both phonon therm ...
... be conditioned by the relaxation of the excited electrons. The nonlinear symmetric perturbation of the tensor extends over a longer time span, and experiences a drastic change of its spectral dependence within the first 1.5 ps, the analysis of which led us to attribute its decay to both phonon therm ...
Chapter 17: Molecular Interactions
... van der Waals interaction, an interaction between closed-shell molecules that varies with separation as 1/r6. 17.5 Interactions between dipoles multipole, an array of point charges. n-pole, an array of point charges with an n-pole moment but no lower moment. monopole, a point charge. quadru ...
... van der Waals interaction, an interaction between closed-shell molecules that varies with separation as 1/r6. 17.5 Interactions between dipoles multipole, an array of point charges. n-pole, an array of point charges with an n-pole moment but no lower moment. monopole, a point charge. quadru ...
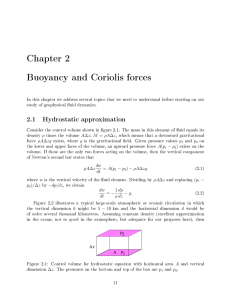
Chapter 2 Buoyancy and Coriolis forces
... ρ times the volume A∆z ; M = ρA∆z , which means that a downward gravitational force ρA∆zg exists, where g is the gravitational eld. Given pressure values p1 and p2 on the lower and upper faces of the volume, an upward pressure force A(p1 − p2 ) exists on the density ...
... ρ times the volume A∆z ; M = ρA∆z , which means that a downward gravitational force ρA∆zg exists, where g is the gravitational eld. Given pressure values p1 and p2 on the lower and upper faces of the volume, an upward pressure force A(p1 − p2 ) exists on the density ...
The Intensity of Ligand Absorption - TopSCHOLAR
... symmetry of these molecules is temporarily removed by an unsymmetrical vibration. Tnese transitions do take place when the electronic transition occurs with simultaneous excitation of one or more vibrational modes (Vibronic Coupling) but with only about 10 J times the intensity of electronically all ...
... symmetry of these molecules is temporarily removed by an unsymmetrical vibration. Tnese transitions do take place when the electronic transition occurs with simultaneous excitation of one or more vibrational modes (Vibronic Coupling) but with only about 10 J times the intensity of electronically all ...
Balancing Chemical Equations
... We can use the mass (weight) of a molecule as one constraint on the formula of a compound (molecule). Some molecules have a simpler empirical formula, which does not correspond to the molecular formula. For example, all carbohydrates have the empirical formula (CH2O). However, the various sugars and ...
... We can use the mass (weight) of a molecule as one constraint on the formula of a compound (molecule). Some molecules have a simpler empirical formula, which does not correspond to the molecular formula. For example, all carbohydrates have the empirical formula (CH2O). However, the various sugars and ...
Physical Chemistry I – review guide
... their initial states • P-V Work is the work done in a volume change, and it can be expressed as ...
... their initial states • P-V Work is the work done in a volume change, and it can be expressed as ...
+ H 2 (g) - UCF Chemistry
... calorimeter. If both solutions were initially at 35.00°C and the temperature of the resulting solution was recorded as 37.00°C, determine the ΔH°rxn (in units of kJ/mol NaOH) for the neutralization reaction between aqueous NaOH and HNO3. Assume 1) that no heat is lost to the calorimeter or the surro ...
... calorimeter. If both solutions were initially at 35.00°C and the temperature of the resulting solution was recorded as 37.00°C, determine the ΔH°rxn (in units of kJ/mol NaOH) for the neutralization reaction between aqueous NaOH and HNO3. Assume 1) that no heat is lost to the calorimeter or the surro ...
H - Workforce3One
... • We define specific heat capacity (or simply specific heat) as the amount of energy required to raise the temperature of 1 g of a substance by 1 K. Thermochemistry ...
... • We define specific heat capacity (or simply specific heat) as the amount of energy required to raise the temperature of 1 g of a substance by 1 K. Thermochemistry ...
Pdf - Text of NPTEL IIT Video Lectures
... 1 minus T2 that is the temperature of the thermal reservoirs where heat is rejected divided by T1. It is a thermal temperature in thermodynamic absolute scale of the reservoir from where heat is being taken that means etaengine is 0.5 into 1 minus 333 divided by 944 and if we equate this value, this ...
... 1 minus T2 that is the temperature of the thermal reservoirs where heat is rejected divided by T1. It is a thermal temperature in thermodynamic absolute scale of the reservoir from where heat is being taken that means etaengine is 0.5 into 1 minus 333 divided by 944 and if we equate this value, this ...
the work done is
... contains energy, but not heat. Energy is associated with a state; heat is associated with a process. Heat is energy in transition. It is recognized only as it crosses the boundaries of a system. Consider the hot baked potato one more time. The potato contains energy, but this called heat only as it ...
... contains energy, but not heat. Energy is associated with a state; heat is associated with a process. Heat is energy in transition. It is recognized only as it crosses the boundaries of a system. Consider the hot baked potato one more time. The potato contains energy, but this called heat only as it ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.