Fundamentals of ultrasound - ASTL
... Definition: An elastic wave carries changes in stress and velocity. Elastic wave is created by a balance between the forces of inertia and of elastic deformation. Particle motion: elastic wave induced material motion Wavespeed: the propagation speed of the elastic wave Particle velocity is much smal ...
... Definition: An elastic wave carries changes in stress and velocity. Elastic wave is created by a balance between the forces of inertia and of elastic deformation. Particle motion: elastic wave induced material motion Wavespeed: the propagation speed of the elastic wave Particle velocity is much smal ...
Soft X-ray spectroscopy of single sized CdS nanocrystals: size
... shifts of the S 2p core level binding energy. We, therefore, have recorded S 2p photoelectron spectra (XPS) of CdS bulk, the NCs, and the Cd thiolate, which refer the binding energies against each other. These XPS spectra will be thoroughly discussed in a forthcoming publication [18], while here we ...
... shifts of the S 2p core level binding energy. We, therefore, have recorded S 2p photoelectron spectra (XPS) of CdS bulk, the NCs, and the Cd thiolate, which refer the binding energies against each other. These XPS spectra will be thoroughly discussed in a forthcoming publication [18], while here we ...
T - Himastron
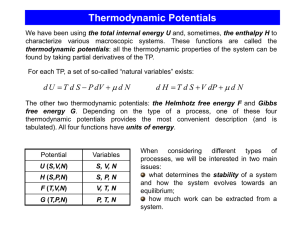
... approach. The entropy, along with V and N, determines the system’s energy U =U (S,V,N). Among the three variable, the entropy is the most difficult to control (the entropy-meters do not exist!). For an isolated system, we have to work with the entropy – it cannot be replaced with some other function ...
... approach. The entropy, along with V and N, determines the system’s energy U =U (S,V,N). Among the three variable, the entropy is the most difficult to control (the entropy-meters do not exist!). For an isolated system, we have to work with the entropy – it cannot be replaced with some other function ...
Work and Energy, Principle of Work and Energy, Principle of Work
... Note that the principle of work and energy (T1 + U1-2 = T2) is not a vector equation! Each term results in a scalar value Both kinetic energy and work have the same units, that of energy! In the SI system, the unit for energy is called a joule (J), where 1 J = 1 N·m. In the FPS system, units are f ...
... Note that the principle of work and energy (T1 + U1-2 = T2) is not a vector equation! Each term results in a scalar value Both kinetic energy and work have the same units, that of energy! In the SI system, the unit for energy is called a joule (J), where 1 J = 1 N·m. In the FPS system, units are f ...
First-principles study of electronic, optical and thermoelectric
... study of these materials has suffered due to the synthesis complications resulting from the thermal instability of Ag+ ions at high temperature. High sintering temperature is required for AgTaO3 , while different combination of electrical and mechanical properties can be achieved from AgNbO3 and simil ...
... study of these materials has suffered due to the synthesis complications resulting from the thermal instability of Ag+ ions at high temperature. High sintering temperature is required for AgTaO3 , while different combination of electrical and mechanical properties can be achieved from AgNbO3 and simil ...
State of Equilibrium
... comprising System A and heat engine ER,was derived. It was also stipulated that System A could change its volume by SV, and while it is doing this it must perform work on the atmosphere equivalent to p o SV, where p o is the pressure of the atmosphere. This work detracts from the work previously cal ...
... comprising System A and heat engine ER,was derived. It was also stipulated that System A could change its volume by SV, and while it is doing this it must perform work on the atmosphere equivalent to p o SV, where p o is the pressure of the atmosphere. This work detracts from the work previously cal ...
THE FREE ENERGIES OF FORMATION OF AQUEOUS d
... The employment of thermodynamics in biochemistry has been restricted, until recently, to the use of first law data. In the last few years a beginning has been made in the application of the second law; i.e., of free energy data (1, 2). The development of this field is limited by the paucity of avail ...
... The employment of thermodynamics in biochemistry has been restricted, until recently, to the use of first law data. In the last few years a beginning has been made in the application of the second law; i.e., of free energy data (1, 2). The development of this field is limited by the paucity of avail ...
Chapter 5 Energy Relationships in Chemistry: Thermochemistry
... calorimeter. If both solutions were initially at 35.00°C and the temperature of the resulting solution was recorded as 37.00°C, determine the ΔH°rxn (in units of kJ/mol NaOH) for the neutralization reaction between aqueous NaOH and HNO3. Assume 1) that no heat is lost to the calorimeter or the surro ...
... calorimeter. If both solutions were initially at 35.00°C and the temperature of the resulting solution was recorded as 37.00°C, determine the ΔH°rxn (in units of kJ/mol NaOH) for the neutralization reaction between aqueous NaOH and HNO3. Assume 1) that no heat is lost to the calorimeter or the surro ...
CYL110 2012-2013 Classical Thermodynamics Sample Problems
... expand isothermally until its final pressure is 1 atm. Calculate the work done if the expansion is carried out (a) against a vacuum, (b) against a constant external pressure of 1 atm, and (c) reversibly. (d) Calculate also the work done if the same process is carried out adiabatically and reversibly ...
... expand isothermally until its final pressure is 1 atm. Calculate the work done if the expansion is carried out (a) against a vacuum, (b) against a constant external pressure of 1 atm, and (c) reversibly. (d) Calculate also the work done if the same process is carried out adiabatically and reversibly ...
Basic Thermodynamics Prof. S. K. Som Department of Mechanical
... All types of energy stored within this system apart from the intermolecular energy which is because of the molecular kinetic and potential energy can be described in terms of microscopic potential energy. ...
... All types of energy stored within this system apart from the intermolecular energy which is because of the molecular kinetic and potential energy can be described in terms of microscopic potential energy. ...
Max Planck: the reluctant revolutionary
... nomena. All the same, his attitude to atomism remained He used it to define the entropy of an ideal oscillator (dipole) ambiguous and he continued to give priority to macroscopic but was careful not to identify such oscillators with specific thermodynamics and ignore Boltzmann's statistical theory. ...
... nomena. All the same, his attitude to atomism remained He used it to define the entropy of an ideal oscillator (dipole) ambiguous and he continued to give priority to macroscopic but was careful not to identify such oscillators with specific thermodynamics and ignore Boltzmann's statistical theory. ...
Slide 1
... pendulum depends on the length of the string, the mass of the pendulum bob, and the amplitude of the swing. It's easy to measure the period using the photogate timer. You can vary friction and the strength of gravity. Use the pendulum to find the value of g on planet X. Notice the anharmonic behavio ...
... pendulum depends on the length of the string, the mass of the pendulum bob, and the amplitude of the swing. It's easy to measure the period using the photogate timer. You can vary friction and the strength of gravity. Use the pendulum to find the value of g on planet X. Notice the anharmonic behavio ...
thermodynamics
... temperatures equalise; the two bodies are then in thermal equilibrium. We saw in some detail how to construct temperature scales to assign temperatures to different bodies. We now describe the concepts of heat and other relevant quantities like internal energy and work. The concept of internal energ ...
... temperatures equalise; the two bodies are then in thermal equilibrium. We saw in some detail how to construct temperature scales to assign temperatures to different bodies. We now describe the concepts of heat and other relevant quantities like internal energy and work. The concept of internal energ ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.