The effect of heat on the metallurgical structure and B
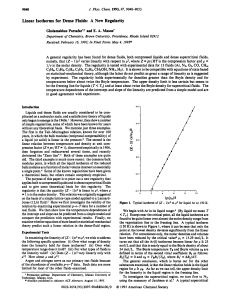
... The differences between B − H hysteresis curves measured before and after heat application show the effect of heat application during melting (burning) on the magnetic characteristics of the stator material. The difference between the two curves can be seen both on the size of the area bordered by the ...
... The differences between B − H hysteresis curves measured before and after heat application show the effect of heat application during melting (burning) on the magnetic characteristics of the stator material. The difference between the two curves can be seen both on the size of the area bordered by the ...
Linear Isotherms for Dense Fluids
... years, in which the bulk modulus (reciprocal compressibility) of a liquid (or solid) is linear in the pressure.2 The second is the linear relation between temperature and density at unit compression factor ( Z = p v / R T = l), discoveredempirically in 1906, then forgotten and rediscovered several t ...
... years, in which the bulk modulus (reciprocal compressibility) of a liquid (or solid) is linear in the pressure.2 The second is the linear relation between temperature and density at unit compression factor ( Z = p v / R T = l), discoveredempirically in 1906, then forgotten and rediscovered several t ...
Principles of Energy Conversion
... storage mechanisms: gas cylinder, propane tank, piston-cylinder, . . . • chemical potential: (internal energy, enthalpy in thermodynamics) storage mechanisms: batteries, coal, petroleum, hydrogen, glucose, . . . • thermal: (sensible & latent heat) storage mechanisms: mass, phase-change material (PCM ...
... storage mechanisms: gas cylinder, propane tank, piston-cylinder, . . . • chemical potential: (internal energy, enthalpy in thermodynamics) storage mechanisms: batteries, coal, petroleum, hydrogen, glucose, . . . • thermal: (sensible & latent heat) storage mechanisms: mass, phase-change material (PCM ...
The Photographic Latent Image From an old Kodak web page As
... This curve departs significantly from linearity only when the exposure becomes so great that appreciable energy is wasted on grains that have already been exposed. For commercially available fine-grain x-ray films, for example, the density versus exposure curve may be essentially linear up to densit ...
... This curve departs significantly from linearity only when the exposure becomes so great that appreciable energy is wasted on grains that have already been exposed. For commercially available fine-grain x-ray films, for example, the density versus exposure curve may be essentially linear up to densit ...
Oxide-ceramic products for high-temperature technology
... Oxide-ceramic materials are often subject to corrosive stresses caused by melting and gases, e.g. during glass processing. Apart from the two extremes – no corrosion attack and complete destruction of material in a very short time – selective attacks on the grain boundary phase must be emphasised. T ...
... Oxide-ceramic materials are often subject to corrosive stresses caused by melting and gases, e.g. during glass processing. Apart from the two extremes – no corrosion attack and complete destruction of material in a very short time – selective attacks on the grain boundary phase must be emphasised. T ...
Pdf - Text of NPTEL IIT Video Lectures
... So, that will be given as half I p and theta dot square, because it is oscillating with theta and velocity is theta dot. So, we can see that both the linear and rotational kinetic energy is there in this. And now we are having a displacement or the extension of the spring because of this displaceme ...
... So, that will be given as half I p and theta dot square, because it is oscillating with theta and velocity is theta dot. So, we can see that both the linear and rotational kinetic energy is there in this. And now we are having a displacement or the extension of the spring because of this displaceme ...
plumbum thiogallate optical properties
... compound has the M1–3xLn2xGa2S4 form, x < 1/3 and x ~ 0.005 for laser crystals. Paper [5] notes that cationic lattice of M ions consists of two sublattices. Half of the cations occupies positions with point group of symmetry D2, while the other half occupies positions with lower symmetry (either C2, ...
... compound has the M1–3xLn2xGa2S4 form, x < 1/3 and x ~ 0.005 for laser crystals. Paper [5] notes that cationic lattice of M ions consists of two sublattices. Half of the cations occupies positions with point group of symmetry D2, while the other half occupies positions with lower symmetry (either C2, ...
Chapter 7: Conservation Laws
... form called internal energy. Internal energy gets its name from the fact that it is energy that seems to be hidden inside materials. We cannot measure it by any external measurement, such as height or speed; only careful measurements of the state of the material itself reveal internal energy. Therma ...
... form called internal energy. Internal energy gets its name from the fact that it is energy that seems to be hidden inside materials. We cannot measure it by any external measurement, such as height or speed; only careful measurements of the state of the material itself reveal internal energy. Therma ...
g - Haiku
... Analyze We need to relate the potential energy of the bowling ball to its position relative to the ground. We then need to establish the relationship between work and the change in the ball’s potential energy. Finally, we need to connect the change in potential energy when the ball is dropped with t ...
... Analyze We need to relate the potential energy of the bowling ball to its position relative to the ground. We then need to establish the relationship between work and the change in the ball’s potential energy. Finally, we need to connect the change in potential energy when the ball is dropped with t ...
Charge-density analysis of an iron–sulfur protein at an ultra
... maps and refinement calculations are performed under strong restraints1,2. Therefore, we usually supplement the information on hydrogen atoms and valence electrons in proteins with preexisting common knowledge obtained by chemistry in small molecules. However, even now, computational calculation of ...
... maps and refinement calculations are performed under strong restraints1,2. Therefore, we usually supplement the information on hydrogen atoms and valence electrons in proteins with preexisting common knowledge obtained by chemistry in small molecules. However, even now, computational calculation of ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.