Nuclear Chemistry - Mrs. Carlyle`s Classroom
... Half-lives can be short as a fraction of a second or as long as several million years. Artificial radioisotopes have very short half-lives. Good for nuclear medicine. It is possible to use this method to date rocks as old as our solar system. ...
... Half-lives can be short as a fraction of a second or as long as several million years. Artificial radioisotopes have very short half-lives. Good for nuclear medicine. It is possible to use this method to date rocks as old as our solar system. ...
radiation!!! - Mr Schmitt
... When these nuclei lose energy and break apart, decay occurs ▪ Radioactive decay releases energy from the nucleus as radiation ▪ Radioactive atoms release energy until they become stable, often ending up as different atoms ▪ For example: uranium-238 (parent nucleus) decays in several stages until i ...
... When these nuclei lose energy and break apart, decay occurs ▪ Radioactive decay releases energy from the nucleus as radiation ▪ Radioactive atoms release energy until they become stable, often ending up as different atoms ▪ For example: uranium-238 (parent nucleus) decays in several stages until i ...
Tips for Taking Levothyroxine
... Whether those with hypothyroidism should avoid soy has long been a topic of debate. It is thought that soy interferes with the bodies ability to absorb Levothyroxine. However, the evidence is not strong enough to suggest people should avoid soy altogether. ...
... Whether those with hypothyroidism should avoid soy has long been a topic of debate. It is thought that soy interferes with the bodies ability to absorb Levothyroxine. However, the evidence is not strong enough to suggest people should avoid soy altogether. ...
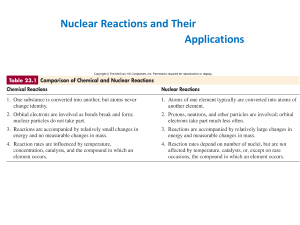
Nuclear Reactions and Their Applications
... biologically like calcium, the element above it in Group 2A(2). When 90Sr is ingested by mammals, it is found in their milk and eventually in the bones of those drinking the milk. If a sample of 90Sr has an activity of 1.2 x 1012 d/s, what are the activity and the fraction of nuclei that have decaye ...
... biologically like calcium, the element above it in Group 2A(2). When 90Sr is ingested by mammals, it is found in their milk and eventually in the bones of those drinking the milk. If a sample of 90Sr has an activity of 1.2 x 1012 d/s, what are the activity and the fraction of nuclei that have decaye ...
Endocrine System Diseases and Disorders
... Pituitary Dwarfism •Hypo GH before 25 •aka proportional dwarfism ...
... Pituitary Dwarfism •Hypo GH before 25 •aka proportional dwarfism ...
Radioactivity - Miami Beach Senior High School
... • The nucleus splits into two smaller nuclei, which are radioactive. • More neutrons are released. • The additional neutrons released may also hit other uranium nuclei and cause them to split. Even more neutrons are then released, which in turn can split more uranium nuclei. This is called a chain r ...
... • The nucleus splits into two smaller nuclei, which are radioactive. • More neutrons are released. • The additional neutrons released may also hit other uranium nuclei and cause them to split. Even more neutrons are then released, which in turn can split more uranium nuclei. This is called a chain r ...
Radioactivity
... • The proton number (or atomic number) Z of an element is the number of protons in each nucleus of the atoms of the element. All its nuclei have the same proton number • The nucleon number (or mass number) A of an atom is the number of nucleons (protons plus neutrons) in its nucleus • A given elemen ...
... • The proton number (or atomic number) Z of an element is the number of protons in each nucleus of the atoms of the element. All its nuclei have the same proton number • The nucleon number (or mass number) A of an atom is the number of nucleons (protons plus neutrons) in its nucleus • A given elemen ...
aaa - E-Learning/An-Najah National University
... prolactin production in the pituitary. High prolactin levels will inhibit gonadotrophin releasing hormone (GnRH) production in the hypothalamus, which is needed for LH and FSH production in the pituitary, needed for normal ovarian function thus causing amenorrhea. ...
... prolactin production in the pituitary. High prolactin levels will inhibit gonadotrophin releasing hormone (GnRH) production in the hypothalamus, which is needed for LH and FSH production in the pituitary, needed for normal ovarian function thus causing amenorrhea. ...
Surgical importance of Thyroid Gland
... Hypercortisolism can occur from; (1) Adenomas of: - Anterior pituitary ↑ ACTH, adrenal hyperplasia and excess cortisol secretion; Hypothalamus high levels of corticotropin- releasing hormone (CRH), ↑ ACTH release; Tumors “ectopic secretion” of ACTH by a tumors, Adenomas of the adrenal cortex. (lo ...
... Hypercortisolism can occur from; (1) Adenomas of: - Anterior pituitary ↑ ACTH, adrenal hyperplasia and excess cortisol secretion; Hypothalamus high levels of corticotropin- releasing hormone (CRH), ↑ ACTH release; Tumors “ectopic secretion” of ACTH by a tumors, Adenomas of the adrenal cortex. (lo ...
Alpha Subunits
... subunit may support the diagnosis of TSHoma, as may the finding of hyper-or hypo-secretion of other pituitary hormones. Pituitary imaging usually confirms the diagnosis, but should not be undertaken until the appropriate biochemical confirmation has been made. A syndrome of thyroid hormone resistanc ...
... subunit may support the diagnosis of TSHoma, as may the finding of hyper-or hypo-secretion of other pituitary hormones. Pituitary imaging usually confirms the diagnosis, but should not be undertaken until the appropriate biochemical confirmation has been made. A syndrome of thyroid hormone resistanc ...
January 2011 - Premier Community Health
... hormones that affect your heart rate, blood pressure, body temperature, weight and it helps you keep a healthy amount of calcium in your body. It helps set your metabolism, or how your body gets energy from the foods you eat. ...
... hormones that affect your heart rate, blood pressure, body temperature, weight and it helps you keep a healthy amount of calcium in your body. It helps set your metabolism, or how your body gets energy from the foods you eat. ...
Nuclear - PEO Scarborough Chapter
... 2. Negative - release of negatively charged particle called electron and antineutrino. For example, carbon-14 (an unstable isotope of carbon), when subjected to beta decay, forms nitrogen (stable) along with an electron; ...
... 2. Negative - release of negatively charged particle called electron and antineutrino. For example, carbon-14 (an unstable isotope of carbon), when subjected to beta decay, forms nitrogen (stable) along with an electron; ...
Iodine-131 administration and risk of cancer: “Appearances can be
... did not refer to a serious limitation which is present in most articles evaluating the supposed carcinogenic effect of 131I therapy, including all but one of the seven studies selected by this meta-analysis. In fact, all published studies assessed whether 131I administration in patients with benign ...
... did not refer to a serious limitation which is present in most articles evaluating the supposed carcinogenic effect of 131I therapy, including all but one of the seven studies selected by this meta-analysis. In fact, all published studies assessed whether 131I administration in patients with benign ...
Assessment and Management of Patients with Endocrine Disorders Dr Ibraheem Bashayreh 29/11/2010
... allowing abundant colloid into the circulation, with neck pain and fever. Patients typically then become hypothyroid as the pituitary reduces TSH production and the inappropriately released colloid is depleted before resolving to euthyroid. The symptoms are those of hyperthyroidism and hypothyroidis ...
... allowing abundant colloid into the circulation, with neck pain and fever. Patients typically then become hypothyroid as the pituitary reduces TSH production and the inappropriately released colloid is depleted before resolving to euthyroid. The symptoms are those of hyperthyroidism and hypothyroidis ...
Thyroidectomy
... Hypothyroidism: Depending how much thyroid tissue is to be left behind, there may not be enough thyroid tissue to supply the necessary amount of thyroid hormone. When this happens it is necessary to take replacement hormone which is taken as a once daily table for the rest of your life. Hypopara ...
... Hypothyroidism: Depending how much thyroid tissue is to be left behind, there may not be enough thyroid tissue to supply the necessary amount of thyroid hormone. When this happens it is necessary to take replacement hormone which is taken as a once daily table for the rest of your life. Hypopara ...
Subacute Thyroiditis Is Treated Effectively by a Low Dose of
... This study is a valuable clinical contribution to thyroidology because it is the first study that analyzed the response to corticosteroid therapy in a large population of patients with subacute thyroiditis. Treatment with about half of the usually recommended steroid dose was effective in ameliorati ...
... This study is a valuable clinical contribution to thyroidology because it is the first study that analyzed the response to corticosteroid therapy in a large population of patients with subacute thyroiditis. Treatment with about half of the usually recommended steroid dose was effective in ameliorati ...
thyroid gland , parathyroid gland exercise -3
... lower chordates :(1) Neural gland (2) Pharyngeal gill pouch (3) Nerve cord (4) Endostyle 2. If thyroid is removed from tadpole of frog, it will :(1) Die soon (2) Remains tadpole throughout life (3) Grow in to giant frog (4) Grows into dwarf frog 3. The basal metabolic rate (BMR) in body cells is reg ...
... lower chordates :(1) Neural gland (2) Pharyngeal gill pouch (3) Nerve cord (4) Endostyle 2. If thyroid is removed from tadpole of frog, it will :(1) Die soon (2) Remains tadpole throughout life (3) Grow in to giant frog (4) Grows into dwarf frog 3. The basal metabolic rate (BMR) in body cells is reg ...
Guided Lecture Notes
... sites that produce excessive amounts of catecholamines. The radionuclide iobenguane (131I) is injected intravenously, and scans are performed on days 2, 3, and 4. Sometimes only 1 day is needed, and other times imaging is needed on days 6 and 7. Normally tumors and sites of hypersecretion are absent ...
... sites that produce excessive amounts of catecholamines. The radionuclide iobenguane (131I) is injected intravenously, and scans are performed on days 2, 3, and 4. Sometimes only 1 day is needed, and other times imaging is needed on days 6 and 7. Normally tumors and sites of hypersecretion are absent ...
1 - The Pathology Guy
... [1. low blood glucose; 2. mental changes fasting/exercising; 3. relieved by giving glucose] ...
... [1. low blood glucose; 2. mental changes fasting/exercising; 3. relieved by giving glucose] ...
Name
... 3. A rem is the quantity of ionizing radiation that does as much damage to human tissue as 1 roentgen of high-voltage X rays does. 4. Exposure varies from one location to another 5. Some activities add to the amount of nuclear radiation exposure. ...
... 3. A rem is the quantity of ionizing radiation that does as much damage to human tissue as 1 roentgen of high-voltage X rays does. 4. Exposure varies from one location to another 5. Some activities add to the amount of nuclear radiation exposure. ...
Nuclear Chemistry - HCC Learning Web
... • Each isotope has a characteristic half-life. • Half-lives are not affected by temperature, pressure or chemical composition. • Natural radioisotopes tend to have longer halflives than synthetic radioisotopes. ...
... • Each isotope has a characteristic half-life. • Half-lives are not affected by temperature, pressure or chemical composition. • Natural radioisotopes tend to have longer halflives than synthetic radioisotopes. ...
Chapter 7 Worksheet
... production of the unstable uranium-236 isotope in your explanation, as well as all the reactants and products of the reaction. C During the nuclear fission reaction of uranium-235 a uranium-235 atom is bombarded with a neutron. C The nucleus of the uranium-235 absorbs the neutron, increasing the mas ...
... production of the unstable uranium-236 isotope in your explanation, as well as all the reactants and products of the reaction. C During the nuclear fission reaction of uranium-235 a uranium-235 atom is bombarded with a neutron. C The nucleus of the uranium-235 absorbs the neutron, increasing the mas ...
Dr. AASHISH H. PANCHAL
... (d)Moderate thyroid function 12. Hormone responsible for the secretion of milk after parturition (a) ICSH (b)Prolactin (c)ACTH (d)LH 13. In addition to thyroxine (T4), triiodothyronine (T3), thyroid gland produces ______ (a) Thyroid-stimulating hormone (b) Adrenocorticotropic hormone (c)Calcitonin ( ...
... (d)Moderate thyroid function 12. Hormone responsible for the secretion of milk after parturition (a) ICSH (b)Prolactin (c)ACTH (d)LH 13. In addition to thyroxine (T4), triiodothyronine (T3), thyroid gland produces ______ (a) Thyroid-stimulating hormone (b) Adrenocorticotropic hormone (c)Calcitonin ( ...
Iodine-131
Iodine-131 (131I), also loosely and nonspecifically called radioiodine, is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because I-131 is a major uranium, plutonium fission product, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. I-131 is also a major fission product of uranium-233, produced from thorium.Due to its mode of beta decay, iodine-131 is notable for causing mutation and death in cells that it penetrates, and other cells up to several millimeters away. For this reason, high doses of the isotope are sometimes less dangerous than low doses, since they tend to kill thyroid tissues that would otherwise become cancerous as a result of the radiation. For example, children treated with moderate dose of I-131 for thyroid adenomas had a detectable increase in thyroid cancer, but children treated with a much higher dose did not. Likewise, most studies of very-high-dose I-131 for treatment of Graves disease have failed to find any increase in thyroid cancer, even though there is linear increase in thyroid cancer risk with I-131 absorption at moderate doses. Thus, iodine-131 is increasingly less employed in small doses in medical use (especially in children), but increasingly is used only in large and maximal treatment doses, as a way of killing targeted tissues. This is known as ""therapeutic use.""Iodine-131 can be ""seen"" by nuclear medicine imaging techniques (i.e., gamma cameras) whenever it is given for therapeutic use, since about 10% of its energy and radiation dose is via gamma radiation. However, since the other 90% of radiation (beta radiation) causes tissue damage without contributing to any ability to see or ""image"" the isotope, other less-damaging radioisotopes of iodine such as iodine-123 (see isotopes of iodine) are preferred in situations when only nuclear imaging is required. The isotope I-131 is still occasionally used for purely diagnostic (i.e., imaging) work, due to its low expense compared to other iodine radioisotopes. Very small medical imaging doses of I-131 have not shown any increase in thyroid cancer. The low-cost availability of I-131, in turn, is due to the relative ease of creating I-131 by neutron bombardment of natural tellurium in a nuclear reactor, then separating I-131 out by various simple methods (i.e., heating to drive off the volatile iodine). By contrast, other iodine radioisotopes are usually created by far more expensive techniques, starting with reactor radiation of expensive capsules of pressurized xenon gas.Iodine-131 is also one of the most commonly used gamma-emitting radioactive industrial tracer. Radioactive tracer isotopes are injected with hydraulic fracturing fluid to determine the injection profile and location of fractures created by hydraulic fracturing.Much smaller incidental doses of iodine-131 than those used in medical therapeutic procedures, are thought to be the major cause of increased thyroid cancers after accidental nuclear contamination. These cancers happen from residual tissue radiation damage caused by the I-131, and usually appear years after exposure, long after the I-131 has decayed.