Chapter 2
... l subshell, describes the shape of the subshell m number of energy states in a subshell s spin moment Chapter 2- ...
... l subshell, describes the shape of the subshell m number of energy states in a subshell s spin moment Chapter 2- ...
Questions - SMK Raja Perempuan Ipoh
... 2.1 MATTER: (refer text book pg 11) 1. Particle Theory of Matter : Matter is made up of …………………… and ……………….. particles. 2. The tiny particles may be atoms, ……………….. and ………………………. 3. Kinetic Theory of matter : Matter consists of small particles that always collide among each other. The particles mo ...
... 2.1 MATTER: (refer text book pg 11) 1. Particle Theory of Matter : Matter is made up of …………………… and ……………….. particles. 2. The tiny particles may be atoms, ……………….. and ………………………. 3. Kinetic Theory of matter : Matter consists of small particles that always collide among each other. The particles mo ...
Particles and Periodic Table
... The three states of matter The three states of matter are solid, liquid and gas. In chemical equations, the three states of matter are shown as (s), (l) and (g), with (aq) for aqueous solutions. Melting and freezing take place at the melting point, boiling and condensing take place at the boiling po ...
... The three states of matter The three states of matter are solid, liquid and gas. In chemical equations, the three states of matter are shown as (s), (l) and (g), with (aq) for aqueous solutions. Melting and freezing take place at the melting point, boiling and condensing take place at the boiling po ...
Calculating the number of Protons, Neutrons, and Electrons.
... The Atom • Is about….. – Anything that has matter/mass or takes up space. – Made up of three subatomic particles (protons, neutrons, and electrons) ...
... The Atom • Is about….. – Anything that has matter/mass or takes up space. – Made up of three subatomic particles (protons, neutrons, and electrons) ...
Chapter 4: The Structure of the Atom
... With each expulsion of an alpha particle from the atom’s nucleus the atom loses 4 units of mass & 2 protons (+2 charged ...
... With each expulsion of an alpha particle from the atom’s nucleus the atom loses 4 units of mass & 2 protons (+2 charged ...
CHAPTER 8
... take into account when measuring ionization energy. When an electron is removed from an atom, the repulsion among the remaining electrons decreases. Because the nuclear charge remains constant, more energy is needed to remove another electron from the positively charged ion. ...
... take into account when measuring ionization energy. When an electron is removed from an atom, the repulsion among the remaining electrons decreases. Because the nuclear charge remains constant, more energy is needed to remove another electron from the positively charged ion. ...
Document
... INTRODUCTION: The overview of the “Why, Where, and What” of bonding It is important that atoms bond. Why? Because they need to bond in order to make _____________, _______________, and other more complex forms of matter. For example, if atoms didn’t bond, you would be quite thirsty all the time! Yes ...
... INTRODUCTION: The overview of the “Why, Where, and What” of bonding It is important that atoms bond. Why? Because they need to bond in order to make _____________, _______________, and other more complex forms of matter. For example, if atoms didn’t bond, you would be quite thirsty all the time! Yes ...
Unit 3.2 worksheet 4 atomic model of matter
... composition (percentage composition) of a compound is a relative measure of the mass (or weight. Click Here - Movie Star Planet Starcoins Generator. HOW TO BECOME POPULAR ON MSP! Tips and tricks! Hope I help :)) Video Rating: / 5. Click Here - Movie Star Planet. Hi i am writing u to ask what is the ...
... composition (percentage composition) of a compound is a relative measure of the mass (or weight. Click Here - Movie Star Planet Starcoins Generator. HOW TO BECOME POPULAR ON MSP! Tips and tricks! Hope I help :)) Video Rating: / 5. Click Here - Movie Star Planet. Hi i am writing u to ask what is the ...
CHAPTER 8 THE PERIODIC TABLE
... take into account when measuring ionization energy. When an electron is removed from an atom, the repulsion among the remaining electrons decreases. Because the nuclear charge remains constant, more energy is needed to remove another electron from the positively charged ion. ...
... take into account when measuring ionization energy. When an electron is removed from an atom, the repulsion among the remaining electrons decreases. Because the nuclear charge remains constant, more energy is needed to remove another electron from the positively charged ion. ...
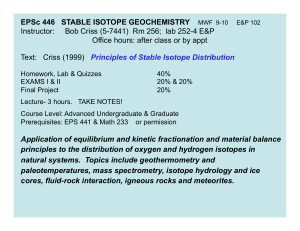
EPSc 446 STABLE ISOTOPE GEOCHEMISTRY Instructor: Bob Criss
... Found that a few α particles were deflected through large angles- up to 180°. ...
... Found that a few α particles were deflected through large angles- up to 180°. ...
Chapter 2 - Chemistry
... ii.) catalyst a substance that speeds up a reaction without undergoing any net change itself - the element symbol of the catalytic substance is often placed over the arrow 2 H2O2 (aq) ...
... ii.) catalyst a substance that speeds up a reaction without undergoing any net change itself - the element symbol of the catalytic substance is often placed over the arrow 2 H2O2 (aq) ...
Fall Exam 4 - Chemistry - University of Kentucky
... He theorized that atoms combine in simple, whole-number ratios to form compounds. He used the oil drop experiment to measure the charge of the electron. ...
... He theorized that atoms combine in simple, whole-number ratios to form compounds. He used the oil drop experiment to measure the charge of the electron. ...
Atomic Number
... Atomic Number The atomic number • is a whole number specific for each element. • is the same for all atoms of an element. • is equal to the number of protons in an atom. • appears above the symbol of an element in the periodic table. Atomic number ...
... Atomic Number The atomic number • is a whole number specific for each element. • is the same for all atoms of an element. • is equal to the number of protons in an atom. • appears above the symbol of an element in the periodic table. Atomic number ...
Mass Number, A
... • Bohr Model or the Solar System Model • Niels Bohr in 1913 introduced his model of the hydrogen atom. • Electrons circle the nucleus in orbits, which are also called energy levels. • An electron can “jump” from a lower energy level to a higher one upon absorbing energy, creating an excited ...
... • Bohr Model or the Solar System Model • Niels Bohr in 1913 introduced his model of the hydrogen atom. • Electrons circle the nucleus in orbits, which are also called energy levels. • An electron can “jump” from a lower energy level to a higher one upon absorbing energy, creating an excited ...
1 - Atomic Theory - Crestwood Local Schools
... 2. Atoms of the same element are identical. 3. The atoms of one element are different from the atoms of another element. 4. Atoms combine in simple whole-number ratios. 5. Atoms are separated, joined or rearranged in chemical reactions. Atoms of one element are never changed into atoms of another el ...
... 2. Atoms of the same element are identical. 3. The atoms of one element are different from the atoms of another element. 4. Atoms combine in simple whole-number ratios. 5. Atoms are separated, joined or rearranged in chemical reactions. Atoms of one element are never changed into atoms of another el ...
CHEM 120 WEEK 11 LECTURES (INORGANIC WEEK 2) Dr. MD
... Contains only metals, apart from boron. Boron is also the only element which does not form a stable trication (B3+) again will have too high a charge density to be stable. Why do the other elements form tri-cations (M3+ )? Soln. √ Because they have the valence electronic configuration ns2np1 and ...
... Contains only metals, apart from boron. Boron is also the only element which does not form a stable trication (B3+) again will have too high a charge density to be stable. Why do the other elements form tri-cations (M3+ )? Soln. √ Because they have the valence electronic configuration ns2np1 and ...
Atoms and Elements
... indivisible, spherical particles made up all of matter. He also argued that these particles were rearranged during a chemical reaction. ...
... indivisible, spherical particles made up all of matter. He also argued that these particles were rearranged during a chemical reaction. ...
Chapter 6 ppt
... • Protons, neutrons, and electrons make up atoms. • All atoms of a given element have the same number of protons in the nucleus. • Isotopes of an element differ by the number of neutrons in the nucleus. • Atomic mass is an average of the masses of all of the naturally occurring isotopes of an elemen ...
... • Protons, neutrons, and electrons make up atoms. • All atoms of a given element have the same number of protons in the nucleus. • Isotopes of an element differ by the number of neutrons in the nucleus. • Atomic mass is an average of the masses of all of the naturally occurring isotopes of an elemen ...
Chapter 2 - The Chemical Context of Life
... Atoms with incomplete valence shells can share or transfer valence electrons with certain other atoms These interactions usually result in atoms staying close together, held by attractions called chemical bonds ...
... Atoms with incomplete valence shells can share or transfer valence electrons with certain other atoms These interactions usually result in atoms staying close together, held by attractions called chemical bonds ...
Study Guide for Electrons Mini-Test - seys
... Periodic table: A tabular arrangement of the elements according to their atomic numbers so that elements with similar properties are in the same column Group: a vertical column in the periodic table = elements in a group share similar properties Period: a horizontal row in the periodic table = prop ...
... Periodic table: A tabular arrangement of the elements according to their atomic numbers so that elements with similar properties are in the same column Group: a vertical column in the periodic table = elements in a group share similar properties Period: a horizontal row in the periodic table = prop ...