Chapter 5 - apel slice
... made of even smaller particles. Thomson was studying the passage of an electric current through a gas. The gas gave off rays that Thomson showed were made of negatively charged particles. But the gas was known to be made of uncharged atoms. So where had the negatively charged particles come from? Fr ...
... made of even smaller particles. Thomson was studying the passage of an electric current through a gas. The gas gave off rays that Thomson showed were made of negatively charged particles. But the gas was known to be made of uncharged atoms. So where had the negatively charged particles come from? Fr ...
Avogadro`s Law is relation between
... c. a main group element in period 3, group 7A d. a main group element in period 2, group 2A e. a halogen in period 2 f. an inner transition metal with one 4f electron 6- Identify the element that fi ts each description. a. an alkaline earth element in period 3 b. a noble gas in period 6 c. a main gr ...
... c. a main group element in period 3, group 7A d. a main group element in period 2, group 2A e. a halogen in period 2 f. an inner transition metal with one 4f electron 6- Identify the element that fi ts each description. a. an alkaline earth element in period 3 b. a noble gas in period 6 c. a main gr ...
Isi dengan Judul Halaman Terkait Adaptif
... Student understands and solvent of description of proton, neutron and electron based on relative charge and his(its relative mass Solvent student of description of mass and charge in atom Solvent student of description of contribution of proton and neutron at atomic nucleus based on atomic num ...
... Student understands and solvent of description of proton, neutron and electron based on relative charge and his(its relative mass Solvent student of description of mass and charge in atom Solvent student of description of contribution of proton and neutron at atomic nucleus based on atomic num ...
1 • Introduction The Scientific Method (1 of 20) 1
... Accuracy refers to how close a measurement is to some accepted or true value (a standard). Ex: an experimental value of the density of Al° is 2.69 g/mL. The accepted value is 2.70 g/mL. Your value is accurate to within 0.37% % error is used to express accuracy. Precision refers to the reliability, r ...
... Accuracy refers to how close a measurement is to some accepted or true value (a standard). Ex: an experimental value of the density of Al° is 2.69 g/mL. The accepted value is 2.70 g/mL. Your value is accurate to within 0.37% % error is used to express accuracy. Precision refers to the reliability, r ...
Early Ideas About Matter
... a chemical reaction. Dalton’s atomic theory easily explains that the con servation of mass in chemical reactions is the result of the separation, combination, or rearrangement of atoms—atoms that are not created, destroyed or divided in the process. The formation of a compound from the combining of ...
... a chemical reaction. Dalton’s atomic theory easily explains that the con servation of mass in chemical reactions is the result of the separation, combination, or rearrangement of atoms—atoms that are not created, destroyed or divided in the process. The formation of a compound from the combining of ...
File
... The nucleus is very small, but it is densely packed with matter. The SI unit for the mass of an atom is the atomic mass unit (amu). One atomic mass unit equals the mass of a proton, which is about 1.7 ⇥ 10 24 g. Each neutron also has a mass of 1 amu. Therefore, the sum of the protons and neutrons in ...
... The nucleus is very small, but it is densely packed with matter. The SI unit for the mass of an atom is the atomic mass unit (amu). One atomic mass unit equals the mass of a proton, which is about 1.7 ⇥ 10 24 g. Each neutron also has a mass of 1 amu. Therefore, the sum of the protons and neutrons in ...
chemistry intro and lesson 1
... second energy level and eight in the third energy level. We will not study the structure of atoms with more than three levels of energy levels. Each energy level must be filled before electrons occupy the next one. Electrons are so light they are considered to have zero mass. Electrons have a negati ...
... second energy level and eight in the third energy level. We will not study the structure of atoms with more than three levels of energy levels. Each energy level must be filled before electrons occupy the next one. Electrons are so light they are considered to have zero mass. Electrons have a negati ...
FINAL REVIEW - Normal Community High School Chemistry
... the burning object has too little oxygen in it, the object will no longer be able to burn. ...
... the burning object has too little oxygen in it, the object will no longer be able to burn. ...
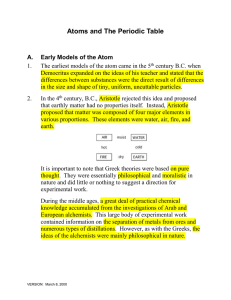
Atoms and The Periodic Table
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
Chemical Bonds - Warren County Public Schools
... Instantly, a vigorous, even violent chemical reaction takes place. The oxygen given off by the heated chlorate rapidly attacks the sugars in the candy, transforming them into carbon dioxide gas and water. The resultant energy is given off as light - a *lot* of light as the case turns out. An ex-Army ...
... Instantly, a vigorous, even violent chemical reaction takes place. The oxygen given off by the heated chlorate rapidly attacks the sugars in the candy, transforming them into carbon dioxide gas and water. The resultant energy is given off as light - a *lot* of light as the case turns out. An ex-Army ...
CHE 128 Autumn 2011 Specific Objectives – Exam 1 A periodic
... Convert units of temperature (˚C, ˚F, K) Calculate the specific heat capacity of a substance – equation will be given Compare specific heat capacities of substances to predict their relative change to heat Predict the nature of electrical charge Identify the names of elements based on their symbols ...
... Convert units of temperature (˚C, ˚F, K) Calculate the specific heat capacity of a substance – equation will be given Compare specific heat capacities of substances to predict their relative change to heat Predict the nature of electrical charge Identify the names of elements based on their symbols ...
Chapter 2. Atoms, Molecules, and Ions - College Test bank
... • Rutherford carried out the following “gold foil” experiment: • A source of -particles was placed at the mouth of a circular detector. • The -particles were shot through a piece of gold foil. • Both the gold nucleus and the -particle were positively charged, so they repelled each other. • Most o ...
... • Rutherford carried out the following “gold foil” experiment: • A source of -particles was placed at the mouth of a circular detector. • The -particles were shot through a piece of gold foil. • Both the gold nucleus and the -particle were positively charged, so they repelled each other. • Most o ...
Unit VIII Atoms… - VernonScienceLSA
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
Chem 151 Chapter 2a
... All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. ...
... All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. ...
Dalton`s Atomic Theory
... Dalton’s atomic theory has been largely accepted by the scientific community, with the exception of three changes. We know now that (1) an atom can be further sub-divided, (2) all atoms of an element are not identical in mass, and (3) using nuclear fission and fusion techniques, we can create or des ...
... Dalton’s atomic theory has been largely accepted by the scientific community, with the exception of three changes. We know now that (1) an atom can be further sub-divided, (2) all atoms of an element are not identical in mass, and (3) using nuclear fission and fusion techniques, we can create or des ...
OXIDATION AND REDUCTION
... Oxidation Number is the valency of an atom in a molecule or ion which is assigned the sign either + or -. It may be (i)electrovalency or (ii)covalency . When the proper sign is associated with valency it becomes oxidation number(ON). It is mostly a theoretical concept particularly for covalent compo ...
... Oxidation Number is the valency of an atom in a molecule or ion which is assigned the sign either + or -. It may be (i)electrovalency or (ii)covalency . When the proper sign is associated with valency it becomes oxidation number(ON). It is mostly a theoretical concept particularly for covalent compo ...
Exam Review 1: CHM 1411 Time: 0hr 55mins
... 1. The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. ...
... 1. The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. ...
Atoms – Building Blocks of Matter Notes
... This idea was widely supported and accepted until the late 1700’s and he too had NO experimental evidence to support his idea. B. Late 1700’s Isaac ____________and Robert ______________ – It was not until the late 1700’s that anyone dared to question Aristotle’s wisdom. They suggested that Aristotle ...
... This idea was widely supported and accepted until the late 1700’s and he too had NO experimental evidence to support his idea. B. Late 1700’s Isaac ____________and Robert ______________ – It was not until the late 1700’s that anyone dared to question Aristotle’s wisdom. They suggested that Aristotle ...
Atom 3 Isotopes - Solon City Schools
... Atoms with the same number of protons & electrons but a different number of neutrons. They are the same element, are chemically identical and undergo the exact same chemical reactions They have different masses (different mass number). All isotopes are used to calculate average atomic mass (this mas ...
... Atoms with the same number of protons & electrons but a different number of neutrons. They are the same element, are chemically identical and undergo the exact same chemical reactions They have different masses (different mass number). All isotopes are used to calculate average atomic mass (this mas ...
Atomic Structure Practice Test
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
Chapter 2 - Chemistry
... 2. All atoms of a given element have the same mass and physical/chemical properties that distinguish them from atoms of other elements. 3. Atoms combine in simple whole number ratios to form compounds. 4. Atoms of one element cannot change into atoms of another element. In chemistry atoms can only c ...
... 2. All atoms of a given element have the same mass and physical/chemical properties that distinguish them from atoms of other elements. 3. Atoms combine in simple whole number ratios to form compounds. 4. Atoms of one element cannot change into atoms of another element. In chemistry atoms can only c ...