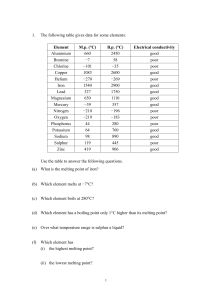
Review for Chapter 8
... 13. Isoelectronic atoms and ions have the same number of electrons and thus the same ground-state electron configuration. For example F-, Ne, and Na+ are isoelectronic with an electron configuration of 1s22s22p6. 14. The concept of effective nuclear charge can be used to explain variations in atomic ...
... 13. Isoelectronic atoms and ions have the same number of electrons and thus the same ground-state electron configuration. For example F-, Ne, and Na+ are isoelectronic with an electron configuration of 1s22s22p6. 14. The concept of effective nuclear charge can be used to explain variations in atomic ...
Review for Midyear - 1 KEY - Ms. Robbins` PNHS Science Classes
... number of protons but a different number of neutrons. Determine the number of protons, neutrons and electrons in Hydrogen-1, hydrogen-2, and hydrogen-3 All have 1 p+ and 1 e- Hydrogen-1 has no neutrons, Hydrogen-2 has 1 neutron, and Hydrogen-3 has 2 neutrons The last digit of an element’s group numb ...
... number of protons but a different number of neutrons. Determine the number of protons, neutrons and electrons in Hydrogen-1, hydrogen-2, and hydrogen-3 All have 1 p+ and 1 e- Hydrogen-1 has no neutrons, Hydrogen-2 has 1 neutron, and Hydrogen-3 has 2 neutrons The last digit of an element’s group numb ...
Bohr-Rutherford Lewis Dot Diagrams Worksheet
... Bohr-Rutherford diagrams are one model that describes what an atom looks like. Consider the atom of lithium. What does the BohrRutherford diagram look like? Step 1: Using the periodic table, calculate the number of protons, neutrons and electrons. Atomic number ...
... Bohr-Rutherford diagrams are one model that describes what an atom looks like. Consider the atom of lithium. What does the BohrRutherford diagram look like? Step 1: Using the periodic table, calculate the number of protons, neutrons and electrons. Atomic number ...
Bohr-Rutherford Lewis Dot Diagrams Worksheet
... Bohr-Rutherford diagrams are one model that describes what an atom looks like. Consider the atom of lithium. What does the Bohr-Rutherford diagram look like? Step 1: Using the periodic table, calculate the number of protons, neutrons and electrons. Atomic number ...
... Bohr-Rutherford diagrams are one model that describes what an atom looks like. Consider the atom of lithium. What does the Bohr-Rutherford diagram look like? Step 1: Using the periodic table, calculate the number of protons, neutrons and electrons. Atomic number ...
Chemistry 1st Grading Period Notes 090211 Pointers Topics Identify
... 3. Light is emitted when an electron jumps from a higher orbit to a lower orbit and absorbed when it jumps from a lower to higher orbit 4. The energy and frequency of light emitted or absorbed is given by the difference between to orbit energies ex: ...
... 3. Light is emitted when an electron jumps from a higher orbit to a lower orbit and absorbed when it jumps from a lower to higher orbit 4. The energy and frequency of light emitted or absorbed is given by the difference between to orbit energies ex: ...
Elements Compounds Mixtures
... • Saturated: all the solute that can be dissolved in a specific amt of solvent at a given temp. – can’t absorb any more. Alloy: Metal solutions that are Solids dissolved in solids— Brass: copper and Zinc Gold: gold and copper ...
... • Saturated: all the solute that can be dissolved in a specific amt of solvent at a given temp. – can’t absorb any more. Alloy: Metal solutions that are Solids dissolved in solids— Brass: copper and Zinc Gold: gold and copper ...
PERIODICITY
... order of increasing atomic number, there is a repetition of their physical and chemical properties. – Elements within a group (column) have similar properties – Properties in a period (row) change as you move from left to right. ...
... order of increasing atomic number, there is a repetition of their physical and chemical properties. – Elements within a group (column) have similar properties – Properties in a period (row) change as you move from left to right. ...
Period Trend
... Trend caused by the increase nuclear charge a higher charge more strongly attracts electrons in the same energy level Group Trends Ionization energies generally decreases down the groups Trend caused by an increase in atomic size as you move down a group with outer electrons farther fr ...
... Trend caused by the increase nuclear charge a higher charge more strongly attracts electrons in the same energy level Group Trends Ionization energies generally decreases down the groups Trend caused by an increase in atomic size as you move down a group with outer electrons farther fr ...
Physical Science Chapter 16 Notes Section 1: Structure of the Atom
... 6. Dalton assumed that all the atoms of the same element would have the same mass, this is not true. We know that atoms of the same elements can have different masses because they can have a different number of neutrons. ♦ Average atomic mass – the average mass of all the isotopes for that element S ...
... 6. Dalton assumed that all the atoms of the same element would have the same mass, this is not true. We know that atoms of the same elements can have different masses because they can have a different number of neutrons. ♦ Average atomic mass – the average mass of all the isotopes for that element S ...
MYP Chemistry: Final Review
... How are elements in the same group (column) related? How are the alkali metals all related? The noble gases? All have the same final electron configuration number; all have same number of valence electrons Alkali Metals: end in s1 configuration, have 1 valence electron Noble gases: end in s2p6, have ...
... How are elements in the same group (column) related? How are the alkali metals all related? The noble gases? All have the same final electron configuration number; all have same number of valence electrons Alkali Metals: end in s1 configuration, have 1 valence electron Noble gases: end in s2p6, have ...
Chapter 4 guided notes (CP)
... Atomic number and Mass number can be found on the periodic table Number of Protons = ____________________________________ Number of electrons = ____________________________________ Number of Neutrons = ____________________________________ ...
... Atomic number and Mass number can be found on the periodic table Number of Protons = ____________________________________ Number of electrons = ____________________________________ Number of Neutrons = ____________________________________ ...
Unit 2
... - alpha particle – two protons and two neutrons bound together and emitted from a nucleus. They are often referred to as helium nuclei particles with a +2 charge. This particle has the lowest penetration of all forms of radiation. - beta particle – an electron emitted from the nucleus during some ki ...
... - alpha particle – two protons and two neutrons bound together and emitted from a nucleus. They are often referred to as helium nuclei particles with a +2 charge. This particle has the lowest penetration of all forms of radiation. - beta particle – an electron emitted from the nucleus during some ki ...
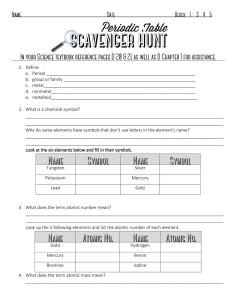
1. Define: a. Period b. group or family
... 12. What feature on the Periodic Table separates the metals and non-metals? _________________________________________________________________________________ 13. Complete the chart below: ...
... 12. What feature on the Periodic Table separates the metals and non-metals? _________________________________________________________________________________ 13. Complete the chart below: ...
Document
... 1. Write the symbol for the metal cation and its charge followed by the symbol for the nonmetal anion and its charge. Obtain charges from the element’s group number in the periodic table (refer ...
... 1. Write the symbol for the metal cation and its charge followed by the symbol for the nonmetal anion and its charge. Obtain charges from the element’s group number in the periodic table (refer ...
Subatomic Particles - Willimon-PHS
... SUBATOMIC PARTICLES https://www.youtube.com/watch?v=FSyAehM dpyI&list=PL8dPuuaLjXtPHzzYuWy6fYEaX9 mQQ8oGr ...
... SUBATOMIC PARTICLES https://www.youtube.com/watch?v=FSyAehM dpyI&list=PL8dPuuaLjXtPHzzYuWy6fYEaX9 mQQ8oGr ...
Concept Reviews Answer sheet Section: matter and Energy 1. a
... has a definite volume and shape. The same substance as a liquid has a definite volume but takes the shape of its container. The same substance as a gas has no definite shape or volume. 3. a 4. c 5. d 6. Either its pressure or volume must also change. Alternatively, both may change. The amplitude and ...
... has a definite volume and shape. The same substance as a liquid has a definite volume but takes the shape of its container. The same substance as a gas has no definite shape or volume. 3. a 4. c 5. d 6. Either its pressure or volume must also change. Alternatively, both may change. The amplitude and ...
Atomic number
... Atomic Numbers of the Elements The atomic number of an element is equal to the number of protons in the nucleus of that element. It gives the identity of the element. Atomic number (Z) = number of protons in nucleus Mass number (A) = number of protons + number of neutrons = atomic number (Z) + numb ...
... Atomic Numbers of the Elements The atomic number of an element is equal to the number of protons in the nucleus of that element. It gives the identity of the element. Atomic number (Z) = number of protons in nucleus Mass number (A) = number of protons + number of neutrons = atomic number (Z) + numb ...
Physical Science Chapter 4 Study Guide mod 5
... 13. What did Thomson use to make his discovery about the atom? Cathode ray 14. Thomson determined that atoms could be divided. 15. Why is it that atoms have no electric charge? They are neutral because they have the same # of protons and electrons 16. According to the modern atomic theory, is it pos ...
... 13. What did Thomson use to make his discovery about the atom? Cathode ray 14. Thomson determined that atoms could be divided. 15. Why is it that atoms have no electric charge? They are neutral because they have the same # of protons and electrons 16. According to the modern atomic theory, is it pos ...
Name: Period: _____ Date
... K. Lewis Diagram L. mass number M. metal N. neutron O. noble gas P. nonmetal Q. orbital R. period S. proton T. semiconductor/metalloid U. valence electron ...
... K. Lewis Diagram L. mass number M. metal N. neutron O. noble gas P. nonmetal Q. orbital R. period S. proton T. semiconductor/metalloid U. valence electron ...