Models of the Atom - Central Magnet School
... • All the positive charge and almost all of the mass are concentrated in a small region in the center of the atom. • He called this tiny core the nucleus. ...
... • All the positive charge and almost all of the mass are concentrated in a small region in the center of the atom. • He called this tiny core the nucleus. ...
Chemical Nomenclature (ionic compounds)
... naming and formulation of compounds. It is universal! The following pages are a programmed approach to the problem of obtaining either the formula or name of a chemical compound. a) The compound will be formed by combining a metal and a non-metal. The metal portion will always appear first in the na ...
... naming and formulation of compounds. It is universal! The following pages are a programmed approach to the problem of obtaining either the formula or name of a chemical compound. a) The compound will be formed by combining a metal and a non-metal. The metal portion will always appear first in the na ...
The Periodic Table
... • Why? The shielding effect makes it easier to remove the outer most electrons as you move down through each family, because the attraction between the valence electrons and the nucleus decreases. ...
... • Why? The shielding effect makes it easier to remove the outer most electrons as you move down through each family, because the attraction between the valence electrons and the nucleus decreases. ...
The Periodic Table and Periodic Law
... – Indicates the relative ability of atoms to attract electrons in a ...
... – Indicates the relative ability of atoms to attract electrons in a ...
CHM 50 Exam 1 Review Name Due Tuesday 9/29/09 Exam 1 will
... Exam 1 will cover chapter 1 – 3. Be sure to study all HW problems and practice problems from the textbook. Your exam will have multiple choices, short answers and solving problems Chapter 1 1. How can a measurement be precise but not accurate? 2. Round off each of the following numbers to three sign ...
... Exam 1 will cover chapter 1 – 3. Be sure to study all HW problems and practice problems from the textbook. Your exam will have multiple choices, short answers and solving problems Chapter 1 1. How can a measurement be precise but not accurate? 2. Round off each of the following numbers to three sign ...
The Periodic Table - Anderson High School
... • Why? The shielding effect makes it easier to remove the outer most electrons as you move down through each family, because the attraction between the valence electrons and the nucleus decreases. ...
... • Why? The shielding effect makes it easier to remove the outer most electrons as you move down through each family, because the attraction between the valence electrons and the nucleus decreases. ...
- Dr.Divan Fard
... B. Sulfur 2) Atomic number 16; 16 protons C. Barium 3) Atomic number 56; 56 protons ...
... B. Sulfur 2) Atomic number 16; 16 protons C. Barium 3) Atomic number 56; 56 protons ...
IPLS Section 4.1 Studying Atoms
... may say that they want to learn more about the structure of atoms. ...
... may say that they want to learn more about the structure of atoms. ...
Electrons
... This atom has gained an electron. Now it has one less proton than electron. One less proton means one less positive charge. This makes the total charge of the atom NEGATIVE. ...
... This atom has gained an electron. Now it has one less proton than electron. One less proton means one less positive charge. This makes the total charge of the atom NEGATIVE. ...
Chapter 4 - H - Regional School District 17
... objects from one location to another. The comparison is an example of an analogy. An analogy uses a similarity to compare two objects or systems. A familiar object is often used to help explain a less familiar object. 1. Atoms in compounds are like bricks in a wall. Explain this analogy. 2. Think of ...
... objects from one location to another. The comparison is an example of an analogy. An analogy uses a similarity to compare two objects or systems. A familiar object is often used to help explain a less familiar object. 1. Atoms in compounds are like bricks in a wall. Explain this analogy. 2. Think of ...
Periodic Table of Elements
... mass starting with Hydrogen (Atomic Mass: 1) and ending with Thorium(Atomic Mass: 56). He found that every eighth element had properties similar to that of the first. He compared this to the octaves found in music. He called it the ‘Law of Octaves’. It is known as ‘Newlands’Law of Octaves’. Advantag ...
... mass starting with Hydrogen (Atomic Mass: 1) and ending with Thorium(Atomic Mass: 56). He found that every eighth element had properties similar to that of the first. He compared this to the octaves found in music. He called it the ‘Law of Octaves’. It is known as ‘Newlands’Law of Octaves’. Advantag ...
Atomic Structure LO Teacher
... 3. Compounds are formed by the joining of atoms of 2 or more elements. In any compound, the atoms of the different elements are joined in a definite, wholenumber ratio, such as 1:1, 2:1, or 3:2. Dalton’s essential ideas are still useful today, but several modifications to his theory have been made… ...
... 3. Compounds are formed by the joining of atoms of 2 or more elements. In any compound, the atoms of the different elements are joined in a definite, wholenumber ratio, such as 1:1, 2:1, or 3:2. Dalton’s essential ideas are still useful today, but several modifications to his theory have been made… ...
Electron Shells - rlas
... electrons are called ______________ electrons. They’re super important, because they are the only electrons that are allowed to _____________ with other atoms. If I ask you how many valence electrons are in an atom, you’ll tell me how many electrons are found in the outermost of all the shells. Poss ...
... electrons are called ______________ electrons. They’re super important, because they are the only electrons that are allowed to _____________ with other atoms. If I ask you how many valence electrons are in an atom, you’ll tell me how many electrons are found in the outermost of all the shells. Poss ...
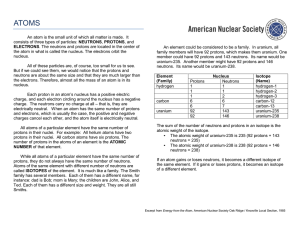
An atom is the small unit of which all matter is made. It consists of
... 5. An isotope of an element gets part of its name from the total number of PROTONS and NEUTRONS in its nuclei. 6. If the isotope helium-4 has 2 protons and 2 neutrons, the isotope helium-6 would have 2 (two) protons and 4 (four) neutrons. 7. One element changes into another element when it gains one ...
... 5. An isotope of an element gets part of its name from the total number of PROTONS and NEUTRONS in its nuclei. 6. If the isotope helium-4 has 2 protons and 2 neutrons, the isotope helium-6 would have 2 (two) protons and 4 (four) neutrons. 7. One element changes into another element when it gains one ...
3lectouttch
... 3. Compounds are formed by the joining of atoms of 2 or more elements. In any compound, the atoms of the different elements are joined in a definite, wholenumber ratio, such as 1:1, 2:1, or 3:2. Dalton’s essential ideas are still useful today, but several modifications to his theory have been made… ...
... 3. Compounds are formed by the joining of atoms of 2 or more elements. In any compound, the atoms of the different elements are joined in a definite, wholenumber ratio, such as 1:1, 2:1, or 3:2. Dalton’s essential ideas are still useful today, but several modifications to his theory have been made… ...
Chap 4 Review with answers
... isotopes: atoms or elements that have the same number of protons in the nucleus but different number of neutrons. ...
... isotopes: atoms or elements that have the same number of protons in the nucleus but different number of neutrons. ...
The Atomic Theory
... Law of Multiple Proportions: If two elements combine to form more than one compound the different weights of one which combine with the same weight of the other are in the ration of small whole numbers. Law of Reciprocal Proportions: The weights of two (or more) substances which react separately wit ...
... Law of Multiple Proportions: If two elements combine to form more than one compound the different weights of one which combine with the same weight of the other are in the ration of small whole numbers. Law of Reciprocal Proportions: The weights of two (or more) substances which react separately wit ...
Physical Science
... solitaire. Mendeleev lined up the elements in order of increasing mass, a pattern emerged. The key was to break the elements into rows. ...
... solitaire. Mendeleev lined up the elements in order of increasing mass, a pattern emerged. The key was to break the elements into rows. ...
Chapter 3 Notes - Scarsdale Schools
... All matter is composed of atoms All atoms of the same element are identical; those of different elements are different Atoms of one element cannot be converted into atoms of another element Atoms unite in definite ratios to form compounds ...
... All matter is composed of atoms All atoms of the same element are identical; those of different elements are different Atoms of one element cannot be converted into atoms of another element Atoms unite in definite ratios to form compounds ...
b. - s3.amazonaws.com
... Strontium is an element that gives a brilliant red color to fireworks. a. In what group is strontium found? b. In what chemical family is strontium found? c. In what period is strontium found? d. What are the name and symbol of the element in Period 3 that is in the same group as strontium? e. What ...
... Strontium is an element that gives a brilliant red color to fireworks. a. In what group is strontium found? b. In what chemical family is strontium found? c. In what period is strontium found? d. What are the name and symbol of the element in Period 3 that is in the same group as strontium? e. What ...
Chapter 4 ppt.
... If an element has an atomic number of 34 and a mass number of 78, what is the: a) number of protons b) number of neutrons c) number of electrons ...
... If an element has an atomic number of 34 and a mass number of 78, what is the: a) number of protons b) number of neutrons c) number of electrons ...
Atoms
... As early as 400 BC scientists have believed in an atomic theory thanks to Democritus. n Atoms were the building blocks of matter. n 2000 years later we can see the atom! n ...
... As early as 400 BC scientists have believed in an atomic theory thanks to Democritus. n Atoms were the building blocks of matter. n 2000 years later we can see the atom! n ...
C C C H1 H H
... The atomic mass unit (amu) is a special unit for measuring the mass of very small particles such as atoms. The relationship between amu and grams is the following: 1.00 amu = 1.66 x 10-24g Note the following diagrams comparing atoms and ions. ...
... The atomic mass unit (amu) is a special unit for measuring the mass of very small particles such as atoms. The relationship between amu and grams is the following: 1.00 amu = 1.66 x 10-24g Note the following diagrams comparing atoms and ions. ...