
Unit 5 Notes
... Radium-226 is a radioactive isotope that decays by releasing an alpha particle. Write a nuclear equation for the radioactive decay of radium-226. ...
... Radium-226 is a radioactive isotope that decays by releasing an alpha particle. Write a nuclear equation for the radioactive decay of radium-226. ...
Atomic Theory - Wappingers Central School District
... - hydrogen had to come from nitrogen - suggested hydrogen nucleus was a particle & named it the proton ...
... - hydrogen had to come from nitrogen - suggested hydrogen nucleus was a particle & named it the proton ...
MID-COURSE REVISION QUESTIONS The following questions are
... arranged within the molecule. For example, the compound hydrogen peroxide has the structure H–O–O–H where each dashed line represents a bonding pair of electrons. Another way of showing this structure without the dashed lines is simply to write it as HOOH. Question 2 (a) Metallic bonding is the char ...
... arranged within the molecule. For example, the compound hydrogen peroxide has the structure H–O–O–H where each dashed line represents a bonding pair of electrons. Another way of showing this structure without the dashed lines is simply to write it as HOOH. Question 2 (a) Metallic bonding is the char ...
Chemistry Midterm Review 2006
... 12. Fill in the chart below. Atomic # Mass # protons electrons neutrons ...
... 12. Fill in the chart below. Atomic # Mass # protons electrons neutrons ...
The Periodic Table
... Elements were listed in columns so that those with similar properties were side by side. ...
... Elements were listed in columns so that those with similar properties were side by side. ...
chapter8
... noble gases behave similarly. This is because all of the elements have completely filled outer ns and np subshells, a condition that represents great stability. The empirical formula are of course, the same as the symbols that represent the elements. Carbon , for example , exists as an extensive th ...
... noble gases behave similarly. This is because all of the elements have completely filled outer ns and np subshells, a condition that represents great stability. The empirical formula are of course, the same as the symbols that represent the elements. Carbon , for example , exists as an extensive th ...
Chapter 2 Expanded Notes
... particles, protons, electrons and neutrons. If every atom has the same three particles, how do they differ from one another? They differ based on the proportion, or mount, of each particle present, not on the ones it has. Electrons are not good for this, since they can be lost or gained by atoms. Ne ...
... particles, protons, electrons and neutrons. If every atom has the same three particles, how do they differ from one another? They differ based on the proportion, or mount, of each particle present, not on the ones it has. Electrons are not good for this, since they can be lost or gained by atoms. Ne ...
Unit 2
... • Gravitational Force-pulls objects toward one another. • Electromagnetic Force-opposite charges are attracted to each other. • Strong Force-holds the protons and neutrons together. • Weak Force-in unstable atoms, a neutron can change into a proton and an electron. ...
... • Gravitational Force-pulls objects toward one another. • Electromagnetic Force-opposite charges are attracted to each other. • Strong Force-holds the protons and neutrons together. • Weak Force-in unstable atoms, a neutron can change into a proton and an electron. ...
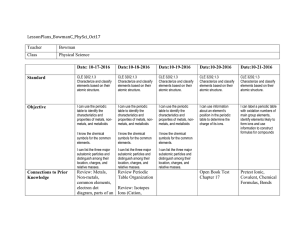
Oct 17-Oct 21
... I can label a periodic table with oxidation numbers of main group elements, identify elements likely to form ions and use information to construct formulas for compounds ...
... I can label a periodic table with oxidation numbers of main group elements, identify elements likely to form ions and use information to construct formulas for compounds ...
Atom
... The atomic mass of copper is 63.546 amu. Which of copper’s two isotopes is more abundant: copper -63 or copper-65? Atomic mass of 63.546 is closer to 63 than 65, thus copper-63 must be more abundant. ...
... The atomic mass of copper is 63.546 amu. Which of copper’s two isotopes is more abundant: copper -63 or copper-65? Atomic mass of 63.546 is closer to 63 than 65, thus copper-63 must be more abundant. ...
Democritus (460
... Planck's quantum theory to explain the stability of most atoms. Bohr suggested the revolutionary idea that electrons "jump" between energy levels (orbits) without ever existing in an in-between state. Thus when an atom absorbs or gives off energy (as in light or heat), the electron jumps to higher o ...
... Planck's quantum theory to explain the stability of most atoms. Bohr suggested the revolutionary idea that electrons "jump" between energy levels (orbits) without ever existing in an in-between state. Thus when an atom absorbs or gives off energy (as in light or heat), the electron jumps to higher o ...
Unit 2: Exploring Matter - Fort McMurray Composite High School
... the elements in order of increasing ATOMIC MASS and created the PERIODIC TABLE - ATOMIC MASS is the average mass of an atom of an element Ex. Oxygen = 16.00 g/mol - Mendeleev found that the properties of the elements repeated at definite, or periodic intervals (ex. Lithium, sodium and potassium have ...
... the elements in order of increasing ATOMIC MASS and created the PERIODIC TABLE - ATOMIC MASS is the average mass of an atom of an element Ex. Oxygen = 16.00 g/mol - Mendeleev found that the properties of the elements repeated at definite, or periodic intervals (ex. Lithium, sodium and potassium have ...
Lap 4: Atomic Structure Mead Chemistry Chapter 4 4.1 Defining the
... Def: Smallest particle of an element that still retains its identity in a chemical reaction Democritus’s Atomic Philosophy Greek philosopher 460 BC -370 BC First to suggest existence of atoms Believed atoms were indivisible and indestructable Ideas proved to be true, but not based on s ...
... Def: Smallest particle of an element that still retains its identity in a chemical reaction Democritus’s Atomic Philosophy Greek philosopher 460 BC -370 BC First to suggest existence of atoms Believed atoms were indivisible and indestructable Ideas proved to be true, but not based on s ...
Molecules Interactive - Avon Community School Corporation
... elements in the human body you copied into your notebook. • Be careful of upper and lower case letters! • After you have listed the symbols, check your answers with a partner, and go to the next slide. ...
... elements in the human body you copied into your notebook. • Be careful of upper and lower case letters! • After you have listed the symbols, check your answers with a partner, and go to the next slide. ...
Document
... • Lived from 384 to 322 BC. • Believed you would never end up with an indivisible particle. • (no such thing as a smallest particle) ...
... • Lived from 384 to 322 BC. • Believed you would never end up with an indivisible particle. • (no such thing as a smallest particle) ...
Document
... stronger than the alkali metals. They also have higher melting points. They are less reactive than alkali metals, but they too are too reactive to be found free in nature. ...
... stronger than the alkali metals. They also have higher melting points. They are less reactive than alkali metals, but they too are too reactive to be found free in nature. ...
OME General Chemistry
... Spraying of oil droplets velocity in presence of gravity noted, then charged droplets were studied in the presence of a known electric field ...
... Spraying of oil droplets velocity in presence of gravity noted, then charged droplets were studied in the presence of a known electric field ...
Structure of Matter
... Atomic Structure ◦ The circular orbits of the Bohr theory are replaced with spherical electron clouds. The wave equations have shown that most electron clouds have shapes that are more complex than Bohr’s orbits but are still simple geometric shapes. ◦ The arrangement of electrons deduced from the ...
... Atomic Structure ◦ The circular orbits of the Bohr theory are replaced with spherical electron clouds. The wave equations have shown that most electron clouds have shapes that are more complex than Bohr’s orbits but are still simple geometric shapes. ◦ The arrangement of electrons deduced from the ...
Unit 2: Exploring Matter
... the elements in order of increasing ATOMIC MASS and created the PERIODIC TABLE - ATOMIC MASS is the average mass of an atom of an element Ex. Oxygen = 16.00 g/mol - Mendeleev found that the properties of the elements repeated at definite, or periodic intervals (ex. Lithium, sodium and potassium have ...
... the elements in order of increasing ATOMIC MASS and created the PERIODIC TABLE - ATOMIC MASS is the average mass of an atom of an element Ex. Oxygen = 16.00 g/mol - Mendeleev found that the properties of the elements repeated at definite, or periodic intervals (ex. Lithium, sodium and potassium have ...
The Periodic Table
... In the modern periodic table elements are arranged in order of increasing atomic number. Periodic Law states: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. ...
... In the modern periodic table elements are arranged in order of increasing atomic number. Periodic Law states: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. ...
Chapter 2
... Most atoms combine chemically with other atoms to form molecules and compounds Molecule—two or more atoms bonded together (e.g., H2 or C6H12O6) Compound—two or more different kinds of atoms bonded together (e.g., C6H12O6) ...
... Most atoms combine chemically with other atoms to form molecules and compounds Molecule—two or more atoms bonded together (e.g., H2 or C6H12O6) Compound—two or more different kinds of atoms bonded together (e.g., C6H12O6) ...
Branches of Chemistry
... Inorganic chemists study the chemistry of all the elements and their compounds, except for those compounds that contain mainly carbon and hydrogen. Nuclear chemists investigate changes that happen in atomic nuclei. Organic chemists study hydrocarbons – compounds of carbon and hydrogen – and other re ...
... Inorganic chemists study the chemistry of all the elements and their compounds, except for those compounds that contain mainly carbon and hydrogen. Nuclear chemists investigate changes that happen in atomic nuclei. Organic chemists study hydrocarbons – compounds of carbon and hydrogen – and other re ...
Unit Expectations – Periodic Table
... C4.10 Neutral Atoms, Ions, and Isotopes - A neutral atom of any element will contain the same number of protons and electrons. Ions are charged particles with an unequal number of protons and electrons. Isotopes are atoms of the same element with different numbers of neutrons and essentially the sam ...
... C4.10 Neutral Atoms, Ions, and Isotopes - A neutral atom of any element will contain the same number of protons and electrons. Ions are charged particles with an unequal number of protons and electrons. Isotopes are atoms of the same element with different numbers of neutrons and essentially the sam ...






















![The atom: Isotopes (Grade 10) [NCS]](http://s1.studyres.com/store/data/016109524_1-1437871a54cd24e5ee13c27e98f0719d-300x300.png)