chemistry 102 fall 2001 part 1
... Directions: (1) Put your name, S.I.D. number and signature on the free response part of the exam where indicated. (2) Each multiple choice question is actually 2 questions on your scanning sheet. If you are sure of an answer, put the same answer down for both questions for 5 pts. If you cannot decid ...
... Directions: (1) Put your name, S.I.D. number and signature on the free response part of the exam where indicated. (2) Each multiple choice question is actually 2 questions on your scanning sheet. If you are sure of an answer, put the same answer down for both questions for 5 pts. If you cannot decid ...
Chem 400 Chem 150 REVIEW SHEET Amanda R
... o Protons, electrons and neutrons make up an atom o Atoms make up molecules, all matter is made of atoms o Protons and neutrons are in the nucleus, and electrons are buzzing outside the nucleus around the nucleus in orbitals o # of protons defines an element, # of protons = atomic # o # of neutrons ...
... o Protons, electrons and neutrons make up an atom o Atoms make up molecules, all matter is made of atoms o Protons and neutrons are in the nucleus, and electrons are buzzing outside the nucleus around the nucleus in orbitals o # of protons defines an element, # of protons = atomic # o # of neutrons ...
Presentation - Chem Rxns - stpats-sch3u-sem1-2013
... collisions and rearrangements of atoms or groups of atoms and that the outcome of collisions depends on the energy and orientation of the collisions ...
... collisions and rearrangements of atoms or groups of atoms and that the outcome of collisions depends on the energy and orientation of the collisions ...
Standard B-2
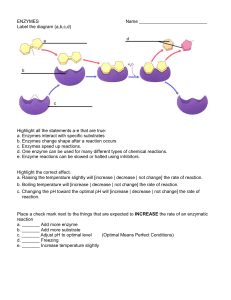
... of a chemical reaction; is not consumed or altered during a chemical reaction, so, it can be used over and over again. o Enzymes: proteins that serve as catalysts in living organisms. o Enzymes are very specific. Each particular enzyme can catalyze only one chemical reaction by working on one partic ...
... of a chemical reaction; is not consumed or altered during a chemical reaction, so, it can be used over and over again. o Enzymes: proteins that serve as catalysts in living organisms. o Enzymes are very specific. Each particular enzyme can catalyze only one chemical reaction by working on one partic ...
Reactions (The Basics)
... How do you know that carbon dioxide was formed? Endothermic or exothermic? ...
... How do you know that carbon dioxide was formed? Endothermic or exothermic? ...
Fun With Predicting Reaction Products
... reaction to occur, both reactants and only one of the products must be soluble in water. If you look up the solubilities on a chart, you’ll find that Ag2SO3 is partly soluble in water, and all of the other compounds are totally soluble in water. This tells us that this reaction will not occur. ...
... reaction to occur, both reactants and only one of the products must be soluble in water. If you look up the solubilities on a chart, you’ll find that Ag2SO3 is partly soluble in water, and all of the other compounds are totally soluble in water. This tells us that this reaction will not occur. ...
Kinetics of the Selective Reaction of Diazonium Salts with Single
... electron w ithdraw ing diazonium salts, w here metallic nanotubes are preferentially functionalised due to their higher electron density at the Fermi level.1 Since this reaction requires the nanotubes to be individualised, it is usually carried out in a surfactant stabilised dispersion. Despite the ...
... electron w ithdraw ing diazonium salts, w here metallic nanotubes are preferentially functionalised due to their higher electron density at the Fermi level.1 Since this reaction requires the nanotubes to be individualised, it is usually carried out in a surfactant stabilised dispersion. Despite the ...
S2-2-07 - Classifying Chemical Reactions
... called a double displacement reaction. Explain reaction type using general formula from notes. Think of other analogies for this type of reaction and fill in notes sheet. Teacher’s analogy: Blackeye + Pinkpanther Blackpanther + Pinkeye. (10 minutes) Have students follow the directions found with m ...
... called a double displacement reaction. Explain reaction type using general formula from notes. Think of other analogies for this type of reaction and fill in notes sheet. Teacher’s analogy: Blackeye + Pinkpanther Blackpanther + Pinkeye. (10 minutes) Have students follow the directions found with m ...
Sample Paper - Army Public School Jammu Cantt
... Use of calculators is not allowed, use log tables wherever required. 1. Name the non stoichiometric point defect responsible for colour in alkali metal halides. 2. What is shape selective catalysis? 3. Amongst the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical ch ...
... Use of calculators is not allowed, use log tables wherever required. 1. Name the non stoichiometric point defect responsible for colour in alkali metal halides. 2. What is shape selective catalysis? 3. Amongst the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical ch ...
CHEM_2nd_Semester_Final_R eview
... 44. Are the following changes of state exothermic or endothermic: a. An ice cube melting c. Water vapor condensing on a mirror b. Dry ice subliming to carbon dioxide d. Water freezing into an ice cube 45. How many calories are needed to raise 450. grams of water from 21.0 oC to 85.5oC? 46. How many ...
... 44. Are the following changes of state exothermic or endothermic: a. An ice cube melting c. Water vapor condensing on a mirror b. Dry ice subliming to carbon dioxide d. Water freezing into an ice cube 45. How many calories are needed to raise 450. grams of water from 21.0 oC to 85.5oC? 46. How many ...
Chemistry 2nd Semester Final Exam Review Chemical Bonds Give
... 44. Are the following changes of state exothermic or endothermic: a. An ice cube melting c. Water vapor condensing on a mirror b. Dry ice subliming to carbon dioxide d. Water freezing into an ice cube 45. How many calories are needed to raise 450. grams of water from 21.0 oC to 85.5oC? 46. How many ...
... 44. Are the following changes of state exothermic or endothermic: a. An ice cube melting c. Water vapor condensing on a mirror b. Dry ice subliming to carbon dioxide d. Water freezing into an ice cube 45. How many calories are needed to raise 450. grams of water from 21.0 oC to 85.5oC? 46. How many ...
Answer Key - La Quinta High School
... takes place. However, the only evidence for this reaction is the release of heat energy, which should be evident as a temperature change for the mixture. Since water has a relatively high specific heat capacity, however, if the acid and base solutions are very dilute, the temperature may change only ...
... takes place. However, the only evidence for this reaction is the release of heat energy, which should be evident as a temperature change for the mixture. Since water has a relatively high specific heat capacity, however, if the acid and base solutions are very dilute, the temperature may change only ...
Document
... steals the date of the other guy. So, a part of one of the reactants trades places and is in a different place among the products. ...
... steals the date of the other guy. So, a part of one of the reactants trades places and is in a different place among the products. ...
Reactions Unit Plan
... 1. Classify the type of chemical reaction as synthesis, decomposition, single replacement, double replacement, and combustion. 2. Predict the products of a chemical reaction using the activity series of metals and solubility rules. B. Apply the Law of Conservation of Mass to writing and balancing ch ...
... 1. Classify the type of chemical reaction as synthesis, decomposition, single replacement, double replacement, and combustion. 2. Predict the products of a chemical reaction using the activity series of metals and solubility rules. B. Apply the Law of Conservation of Mass to writing and balancing ch ...
Cluster Fragmentation and Catalysis
... such, they are usually thought of as bridging the gap between the gas and condensed phases; they can be used as tools for selective microsolvation, or they can be a distinct class of materials with unique properties. Clusters, upon impact with a rigid surface at supersonic velocities, can also catal ...
... such, they are usually thought of as bridging the gap between the gas and condensed phases; they can be used as tools for selective microsolvation, or they can be a distinct class of materials with unique properties. Clusters, upon impact with a rigid surface at supersonic velocities, can also catal ...
Name - rwebbchem
... 1. Would a precipitate form from a reaction of aluminum chloride and sodium hydroxide? If yes, write and balance the equation that illustrates the reaction. ...
... 1. Would a precipitate form from a reaction of aluminum chloride and sodium hydroxide? If yes, write and balance the equation that illustrates the reaction. ...
1 Types of Chemical Reactions
... 2 KI + Pb(NO3)2 See chapter 4, lesson 16 , Saunders Interactive Chemistry ...
... 2 KI + Pb(NO3)2 See chapter 4, lesson 16 , Saunders Interactive Chemistry ...