Biology Fall Semester Test 1 Study Guide
... Two products of cellular respiration are: In producers, chlorophyll and sunlight are necessary for the process of: The closing of its shell when a clam is removed from its watery environment is an example of how a clam maintains its: In a trophic pyramid, _______% of the energy from a source is pass ...
... Two products of cellular respiration are: In producers, chlorophyll and sunlight are necessary for the process of: The closing of its shell when a clam is removed from its watery environment is an example of how a clam maintains its: In a trophic pyramid, _______% of the energy from a source is pass ...
File
... energy is released as bonds are formed. D) Energy is absorbed as bonds are formed, and energy is released as bonds are broken. 4. What occurs in order to break the bond in a Cl2 ...
... energy is released as bonds are formed. D) Energy is absorbed as bonds are formed, and energy is released as bonds are broken. 4. What occurs in order to break the bond in a Cl2 ...
Microbiology: A Systems Approach
... Chemical bonds involve atoms sharing, donating or accepting electrons ...
... Chemical bonds involve atoms sharing, donating or accepting electrons ...
Unit 6 Worksheet Package
... between these two types of ions forms an _____________ bond. Nearly all ionic compounds are _____________ solids at room temperature. In these solids the total _____________ charge is balanced by the total _____________ charge. Ionic compounds in general have very _____________ melting points. This ...
... between these two types of ions forms an _____________ bond. Nearly all ionic compounds are _____________ solids at room temperature. In these solids the total _____________ charge is balanced by the total _____________ charge. Ionic compounds in general have very _____________ melting points. This ...
Chapter 1: Chemistry and You
... Isotopic Notation, Subatomic particles Valence Electrons and Ions 3. Describe the basic structure of the atom in the modern atomic theory (be able to label protons, neutrons, electrons, and the nucleus) (Ch. 3): ...
... Isotopic Notation, Subatomic particles Valence Electrons and Ions 3. Describe the basic structure of the atom in the modern atomic theory (be able to label protons, neutrons, electrons, and the nucleus) (Ch. 3): ...
3rd Quarter Test
... c) concentration of the reactants and the products becomes equal d) rates of the opposing reaction becomes equal 20) For a chemical system at equilibrium, a rise in temperature will a) favor the endothermic reaction b) favor the exothermic reaction c) decrease the rates of reaction d) have no effect ...
... c) concentration of the reactants and the products becomes equal d) rates of the opposing reaction becomes equal 20) For a chemical system at equilibrium, a rise in temperature will a) favor the endothermic reaction b) favor the exothermic reaction c) decrease the rates of reaction d) have no effect ...
Chapters 9 and 10
... a. Consider the carbon dioxide molecule, CO2, and the carbonate ion, CO32-. i. Draw the complete Lewis electron-dot structure for each species. ii. Account for the fact that the carbon-oxygen bond length in CO32- is greater than the carbon-oxygen bond length in CO2. b. Consider the molecules CF4 and ...
... a. Consider the carbon dioxide molecule, CO2, and the carbonate ion, CO32-. i. Draw the complete Lewis electron-dot structure for each species. ii. Account for the fact that the carbon-oxygen bond length in CO32- is greater than the carbon-oxygen bond length in CO2. b. Consider the molecules CF4 and ...
chapter02_part1_lecture - bloodhounds Incorporated
... 2.2 Elements and Compounds • Molecules form when two or more atoms bond together (example: O2) • Compounds form when two or more different elements bond together (H2O) • When a chemical reaction occurs, energy may be given off or absorbed. ...
... 2.2 Elements and Compounds • Molecules form when two or more atoms bond together (example: O2) • Compounds form when two or more different elements bond together (H2O) • When a chemical reaction occurs, energy may be given off or absorbed. ...
Chapter 2 part 1
... 2.2 Elements and Compounds • Molecules form when two or more atoms bond together (example: O2) • Compounds form when two or more different elements bond together (H2O) • When a chemical reaction occurs, energy may be given off or absorbed. ...
... 2.2 Elements and Compounds • Molecules form when two or more atoms bond together (example: O2) • Compounds form when two or more different elements bond together (H2O) • When a chemical reaction occurs, energy may be given off or absorbed. ...
Ground State
... Pieter Zeeman, Lorentz “spectra line splitting” in magnetic filed 1902 Nobel Prize ...
... Pieter Zeeman, Lorentz “spectra line splitting” in magnetic filed 1902 Nobel Prize ...
File - Mr. Gittermann
... • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
... • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
nature of Matter
... Depending on how the electrons interact, the type of bond is decided. The main types of chemical bonds are Ionic & Covalent. When electrons are transferred from one atom to another, an ionic bond is formed. An atom that loses electrons has a + charge. An atom that gains an electron has a – charge. T ...
... Depending on how the electrons interact, the type of bond is decided. The main types of chemical bonds are Ionic & Covalent. When electrons are transferred from one atom to another, an ionic bond is formed. An atom that loses electrons has a + charge. An atom that gains an electron has a – charge. T ...
File
... valence shell) usually determine how an atom will react Atoms are stable when their outer energy level is full Atoms can gain or lose electrons to ...
... valence shell) usually determine how an atom will react Atoms are stable when their outer energy level is full Atoms can gain or lose electrons to ...
Chapter 8: Chemical Reactions and Physical Changes
... • Mass number: total protons and neutrons in an atom’s nucleus • Atomic mass: the average mass of a sample of atoms of that element found in nature • Periodic table: chart that arranges elements by atomic number into rows and columns according to similarities in their properties ...
... • Mass number: total protons and neutrons in an atom’s nucleus • Atomic mass: the average mass of a sample of atoms of that element found in nature • Periodic table: chart that arranges elements by atomic number into rows and columns according to similarities in their properties ...
Atomic Structure 1. Historical perspective of the model of the atom a
... a.) In 1803, John Dalton proposed the atomic theory which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or ...
... a.) In 1803, John Dalton proposed the atomic theory which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or ...
Chemistry Part 1
... Rule of eights – Atoms are considered stable when their outermost orbital has 8 electrons – The exception to this rule of eights is Shell 1, which can only hold 2 electrons ...
... Rule of eights – Atoms are considered stable when their outermost orbital has 8 electrons – The exception to this rule of eights is Shell 1, which can only hold 2 electrons ...
Organic Chemistry
... A. Rules for Drawing Resonance Structures 1. Only electrons move! Nuclei and the sigma- (single bond-) framework are unchanged (Resonance occurs in the pi-system: conjugated lone pairs and pi-bonds). 2. Every resonance structure must be a valid Lewis structure. 3. Keep track of lone pairs and forma ...
... A. Rules for Drawing Resonance Structures 1. Only electrons move! Nuclei and the sigma- (single bond-) framework are unchanged (Resonance occurs in the pi-system: conjugated lone pairs and pi-bonds). 2. Every resonance structure must be a valid Lewis structure. 3. Keep track of lone pairs and forma ...
BASIC CHEMISTRY
... The atomic number for O is 8. How many protons in O? How many electrons in O? The atomic mass of O is 16. How many neutrons in O? Draw an Oxygen atom. Show the number of protons and neutrons in the nucleus and the electrons in the energy ...
... The atomic number for O is 8. How many protons in O? How many electrons in O? The atomic mass of O is 16. How many neutrons in O? Draw an Oxygen atom. Show the number of protons and neutrons in the nucleus and the electrons in the energy ...
Slide 1
... 1. list all the elements follow with an equal sign 2. follow with the number of atoms of that type in the molecule 1. follow with a multiplication sign 2. If the element is O follow with a -2 3. If the element is H follow with a +1 4. any other element enter a ? 5. follow with an = sign, do the math ...
... 1. list all the elements follow with an equal sign 2. follow with the number of atoms of that type in the molecule 1. follow with a multiplication sign 2. If the element is O follow with a -2 3. If the element is H follow with a +1 4. any other element enter a ? 5. follow with an = sign, do the math ...
Chapter 2 Study Guides
... 13. The prefix mono-‐ means “one,” and the prefix poly-‐ means “many.” How are these meanings related to the terms monomer and polymer? ...
... 13. The prefix mono-‐ means “one,” and the prefix poly-‐ means “many.” How are these meanings related to the terms monomer and polymer? ...
Pure Substances and Mixtures
... number of valence electrons can be determined by the column number; 1A has 1 valence electron, IIA has 2 valence electrons, etc. ...
... number of valence electrons can be determined by the column number; 1A has 1 valence electron, IIA has 2 valence electrons, etc. ...
Biochemistry-Review of the Basics
... All atoms want complete electron shells and will bond with others to get them ...
... All atoms want complete electron shells and will bond with others to get them ...
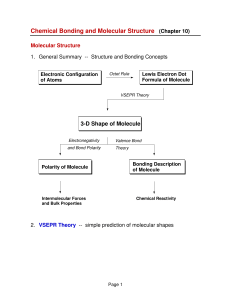
3-D Shape of Molecule
... 2. Molecular Orbitals for simple diatomic molecules (H2 and He2) in H2 the 1s atomic orbitals on the two H atoms are combined into: a bonding MO -- σ1s and an antibonding MO -- σ*1s MO energy level diagram for H2 (only the bonding MO is filled): ...
... 2. Molecular Orbitals for simple diatomic molecules (H2 and He2) in H2 the 1s atomic orbitals on the two H atoms are combined into: a bonding MO -- σ1s and an antibonding MO -- σ*1s MO energy level diagram for H2 (only the bonding MO is filled): ...
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms).Each contributing structure can be represented by a Lewis structure, with only an integer number of covalent bonds between each pair of atoms within the structure. Several Lewis structures are used collectively to describe the actual molecular structure, which is an approximate intermediate between the canonical forms called a resonance hybrid. Contributing structures differ only in the position of electrons, not in the position of nuclei.Electron delocalization lowers the potential energy of the substance and thus makes it more stable than any of the contributing structures. The difference between the potential energy of the actual structure and that of the contributing structure with the lowest potential energy is called the resonance energy or delocalization energy.Resonance is distinguished from tautomerism and conformational isomerism, which involve the formation of isomers, thus the rearrangement of the nuclear positions.