Vocabulary Notes
... Directions: Place the proper vocabulary word on the line on the answer sheet. Vocabulary words can be used more than once. ...
... Directions: Place the proper vocabulary word on the line on the answer sheet. Vocabulary words can be used more than once. ...
Electron-Config
... The chemical properties of atoms, ions, and molecules are related to the arrangement of the ELECTRONS within them. Over time, the atomic model has evolved regarding where electrons are located. ...
... The chemical properties of atoms, ions, and molecules are related to the arrangement of the ELECTRONS within them. Over time, the atomic model has evolved regarding where electrons are located. ...
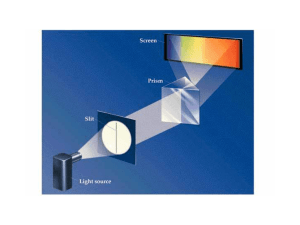
Quantum Mechanics I. Introduction Just before 1900, the classical
... C. Classical theory, modeling the atoms as harmonic oscillators, resulted in the “ultraviolet catastrophe”. Max Planck, in order to reproduce the experimental results, had to assume that each oscillator could only have an integral number of units of energy, rather than have any arbitrary amount. In ...
... C. Classical theory, modeling the atoms as harmonic oscillators, resulted in the “ultraviolet catastrophe”. Max Planck, in order to reproduce the experimental results, had to assume that each oscillator could only have an integral number of units of energy, rather than have any arbitrary amount. In ...
Review for Chapter 3: Atoms, Electrons and Periodic Trends Text
... both the position (energy) of an electron and its momentum (where it is going) at the same time. This meant that Bohr’s model of the atom with the electrons moving in fixed, defined, predictable orbits could not be correct. 4e) Schrodinger pulled together the ideas of all of the above researchers. H ...
... both the position (energy) of an electron and its momentum (where it is going) at the same time. This meant that Bohr’s model of the atom with the electrons moving in fixed, defined, predictable orbits could not be correct. 4e) Schrodinger pulled together the ideas of all of the above researchers. H ...
Unit 1, Lecture 1
... The properties of electrons They are negatively charged. They have a spin (either up or down). The shapes of s and p orbitals s orbitals are spherically symmetric (“round”). p orbitals have two lobes with opposite sign along the axes. p orbitals are also triply degenerate. Atomic energy levels and e ...
... The properties of electrons They are negatively charged. They have a spin (either up or down). The shapes of s and p orbitals s orbitals are spherically symmetric (“round”). p orbitals have two lobes with opposite sign along the axes. p orbitals are also triply degenerate. Atomic energy levels and e ...
Section 4-2 The Quantum Model of the Atom Problems with the Bohr
... D. Atomic Orbitals and Quantum Numbers: Q.N. Specify the properties of atomic orbitals and the properties of electrons in the orbitals I. Principle Quantum Number: “n” a. Indicates the main “energy level” of the electron. b. “n” can only be positive integers beginning with the number 1 c. As “n” in ...
... D. Atomic Orbitals and Quantum Numbers: Q.N. Specify the properties of atomic orbitals and the properties of electrons in the orbitals I. Principle Quantum Number: “n” a. Indicates the main “energy level” of the electron. b. “n” can only be positive integers beginning with the number 1 c. As “n” in ...
Chapter 27
... A current in the filament causes electrons to be emitted These freed electrons are accelerated toward a dense metal target The target is held at a higher potential than the filament ...
... A current in the filament causes electrons to be emitted These freed electrons are accelerated toward a dense metal target The target is held at a higher potential than the filament ...
chemI.final.rev.probs
... 41. For the following reaction: 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) a) Determine the number of grams of KOH that will be produced when 97 g of potassium are used. b) Determine the number of liters of H2 gas that will be produced when 6.5 X 1024 molecules of water are reacted. ...
... 41. For the following reaction: 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) a) Determine the number of grams of KOH that will be produced when 97 g of potassium are used. b) Determine the number of liters of H2 gas that will be produced when 6.5 X 1024 molecules of water are reacted. ...
Name: Period
... scientific method to solve a problem in your life and label each part. 4. Theory v. Scientific Law --- What is the difference? Think of examples for both and identify the limitations. Can they be proved/disproved supported/falsified? ...
... scientific method to solve a problem in your life and label each part. 4. Theory v. Scientific Law --- What is the difference? Think of examples for both and identify the limitations. Can they be proved/disproved supported/falsified? ...
ATOMIC STRUCTURE NOTES n hcZ E ℜ
... An electron is assumed to experience an effective nuclear charge (Zeff) dependent on radius (location), n and l (differing radial distribution functions). The reduction of the true nuclear charge to the effective nuclear charge is called shielding. Zeff = Z – σ. The closer to the nucleus that an ele ...
... An electron is assumed to experience an effective nuclear charge (Zeff) dependent on radius (location), n and l (differing radial distribution functions). The reduction of the true nuclear charge to the effective nuclear charge is called shielding. Zeff = Z – σ. The closer to the nucleus that an ele ...
CHM 101 - Academic Computer Center
... Cold packs, whose temperatures are lowered when ammonium nitrate dissolves in water, are carried by athletic trainers when transporting ice is not possible. Which of the following is true of this reaction? A. H < 0, process is exothermic B. H > 0, process is exothermic C. H < 0, process is endoth ...
... Cold packs, whose temperatures are lowered when ammonium nitrate dissolves in water, are carried by athletic trainers when transporting ice is not possible. Which of the following is true of this reaction? A. H < 0, process is exothermic B. H > 0, process is exothermic C. H < 0, process is endoth ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 18. Illustrate the Pauli Exclusion Principle for the ground state of He atom. 19. At what distance from the nucleus is the probability of finding the electron a maximum for a 1S electron in hydrogen? 20. While the order is the same for both C3v and C3h point groups, their classes are different reaso ...
... 18. Illustrate the Pauli Exclusion Principle for the ground state of He atom. 19. At what distance from the nucleus is the probability of finding the electron a maximum for a 1S electron in hydrogen? 20. While the order is the same for both C3v and C3h point groups, their classes are different reaso ...
Study Guide Answers
... 1. A change of state is a _c__ a. Process by which two states of matter co-exist b. Chemical change c. Physical change that converts a substance from one physical form to another 2. Particles in a ___solid_______ move slower than particles in a ____liquid or gas______. 3. Particles in a _a__ vibrate ...
... 1. A change of state is a _c__ a. Process by which two states of matter co-exist b. Chemical change c. Physical change that converts a substance from one physical form to another 2. Particles in a ___solid_______ move slower than particles in a ____liquid or gas______. 3. Particles in a _a__ vibrate ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.