Exam 1 Topics to Review (McMurry Chpts 1
... 3. Write and interpret isotope symbols for atoms. a. Know atomic number, mass number. b. Find number of protons, neutrons, and electrons from isotope symbol. 4. Isotopes: Calculate atomic mass of an element based on isotopic abundance a. Understand atomic mass unit. ...
... 3. Write and interpret isotope symbols for atoms. a. Know atomic number, mass number. b. Find number of protons, neutrons, and electrons from isotope symbol. 4. Isotopes: Calculate atomic mass of an element based on isotopic abundance a. Understand atomic mass unit. ...
Glossary
... Unit cell − the smallest repeating unit of solids. Units − fundamental and derived standards against which measurements can be made. Fundamental units are defined for mass, length, time, temperature, electric current, luminosity and amount of matter. Derived units include area (length x length), vol ...
... Unit cell − the smallest repeating unit of solids. Units − fundamental and derived standards against which measurements can be made. Fundamental units are defined for mass, length, time, temperature, electric current, luminosity and amount of matter. Derived units include area (length x length), vol ...
N - University of St Andrews
... a 4s electron can get closer to the nucleus than a 3d, i.e. in many-electron atoms the 4s better penetrates the closed shell and its energy decreases. So it turns out that the last electron in potassium is 4s. ...
... a 4s electron can get closer to the nucleus than a 3d, i.e. in many-electron atoms the 4s better penetrates the closed shell and its energy decreases. So it turns out that the last electron in potassium is 4s. ...
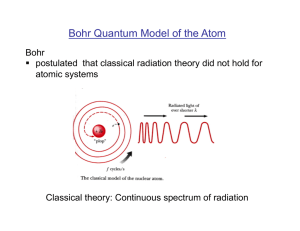
Bohr Quantum Model of the Atom
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
Manne Siegbahn - Nobel Lecture
... completed electron structure of the same type as that of the inert atom next to it, but has in addition a loosely bound valency electron. The optical spectrum is emitted when this loosely bound valency electron moves from one quantum orbit to another. For the X-ray spectra, too, we must assume that ...
... completed electron structure of the same type as that of the inert atom next to it, but has in addition a loosely bound valency electron. The optical spectrum is emitted when this loosely bound valency electron moves from one quantum orbit to another. For the X-ray spectra, too, we must assume that ...
Chapter 8 - Fayetteville State University
... -4A. for a single element complement one another. B. can be used to identify elements in unknown samples, but only if the element is already known by classical chemical means. C. when combined together form a series of bright lines. D. for certain pairs of closely-related elements are identical. 14 ...
... -4A. for a single element complement one another. B. can be used to identify elements in unknown samples, but only if the element is already known by classical chemical means. C. when combined together form a series of bright lines. D. for certain pairs of closely-related elements are identical. 14 ...
Chapter 5
... The energies of atomic orbitals increase as the principal quantum number, n, increases. The energies of the orbitals increase within a shell as the quantum number, , increases. For atomic numbers greater than 20, the relative energies of the orbitals may differ slightly from the order shown. For ex ...
... The energies of atomic orbitals increase as the principal quantum number, n, increases. The energies of the orbitals increase within a shell as the quantum number, , increases. For atomic numbers greater than 20, the relative energies of the orbitals may differ slightly from the order shown. For ex ...
8th Grade Post Physical Science Test Study Guide PS 1: The
... = density = 5 g/mL Mass is measured in grams (base unit), volume measured in , ml, cm3 Units for density: g/cm3, g/mL F. Characteristics of types of matter based on physical and chemical properties. Acids and Bases. Acid: pH below 7, produces hydrogen ions (H+) Base: pH above 7, produces hydroxi ...
... = density = 5 g/mL Mass is measured in grams (base unit), volume measured in , ml, cm3 Units for density: g/cm3, g/mL F. Characteristics of types of matter based on physical and chemical properties. Acids and Bases. Acid: pH below 7, produces hydrogen ions (H+) Base: pH above 7, produces hydroxi ...
Chapter 8 Study Guide
... b. Atoms of a given element are identical in their physical and chemical properties. c. Atoms of different elements differ in their physical and chemical properties. d. Atoms of different elements combine in simple, whole number ratios to form compounds e. In chemical reactions, atoms are combined, ...
... b. Atoms of a given element are identical in their physical and chemical properties. c. Atoms of different elements differ in their physical and chemical properties. d. Atoms of different elements combine in simple, whole number ratios to form compounds e. In chemical reactions, atoms are combined, ...
Chapter 4 - Tolland High School
... • The Bohr model was more accurate than previous models but was only completely accurate for Hydrogen, other elements did not behave exactly as Bohr predicted • The Quantum model was later developed based on work of many scientists including Schrodinger, Heisenberg, & Einstein ...
... • The Bohr model was more accurate than previous models but was only completely accurate for Hydrogen, other elements did not behave exactly as Bohr predicted • The Quantum model was later developed based on work of many scientists including Schrodinger, Heisenberg, & Einstein ...
CH160: Professor Peter Sadler Introduction to inorganic chemistry
... Operator working on a function = (scaler quantity) x (original function) Because H is an operator this is a special equation. The operator contains the sources of energy in the system (kinetic and potential energy) 1927 Heisenberg's uncertainty principle : product of uncertainty in position and unce ...
... Operator working on a function = (scaler quantity) x (original function) Because H is an operator this is a special equation. The operator contains the sources of energy in the system (kinetic and potential energy) 1927 Heisenberg's uncertainty principle : product of uncertainty in position and unce ...
Atomic Theory Notes Packet
... each color observed, record both the color and the wavelength in the table below. The wavelength Using the equation above, calculate the energy values and record in the table. ...
... each color observed, record both the color and the wavelength in the table below. The wavelength Using the equation above, calculate the energy values and record in the table. ...
CHM 2045C - State College of Florida
... This course meets Area V for the A.A./A.S. general education requirements. A rigorous study of chemistry principles for students who have already studied basic concepts of chemistry. This course is intended for science and science-related majors. ...
... This course meets Area V for the A.A./A.S. general education requirements. A rigorous study of chemistry principles for students who have already studied basic concepts of chemistry. This course is intended for science and science-related majors. ...
No Slide Title
... The metals in these two groups have similar outer electron configurations, with one electron in the outermost s orbital. Chemical properties are quite different due to difference in the ionization energy. ...
... The metals in these two groups have similar outer electron configurations, with one electron in the outermost s orbital. Chemical properties are quite different due to difference in the ionization energy. ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.