Chemistry (B) Final Exam Study Guide 1
... c. moves within its atomic orbital d. falls into the nucleus ____ 56. What is the approximate energy of a photon having a frequency of 4 10 Hz? (h = 6.6 10 J s) a. 3 10 J c. 2 10 J b. 3 10 J d. 3 10 J ____ 57. How do the energy differences between the higher energy levels of an atom compare with the ...
... c. moves within its atomic orbital d. falls into the nucleus ____ 56. What is the approximate energy of a photon having a frequency of 4 10 Hz? (h = 6.6 10 J s) a. 3 10 J c. 2 10 J b. 3 10 J d. 3 10 J ____ 57. How do the energy differences between the higher energy levels of an atom compare with the ...
paper 1 - ResearchGate
... d) Suppose the box within which argon translations is 0.01 nm in length. Calculate the n for the particle in the box energy that is equal to kBT/2. e) Based on your answer in part d, how do quantum effects vary with the size of the box a? Do quantum effect increase or decrease with box size? f) Base ...
... d) Suppose the box within which argon translations is 0.01 nm in length. Calculate the n for the particle in the box energy that is equal to kBT/2. e) Based on your answer in part d, how do quantum effects vary with the size of the box a? Do quantum effect increase or decrease with box size? f) Base ...
ChLM Final Review Name: Period: Base Knowledge 1. Classify the
... 1. Classify the following as observations or inferences a) The liquid is green because food coloring was added. b) The beaker has green liquid in it. c) The beaker can hold up to 250 mL. d) The beaker will be the best tool for this lab. 2. Measure the following, circle your estimated digit and inclu ...
... 1. Classify the following as observations or inferences a) The liquid is green because food coloring was added. b) The beaker has green liquid in it. c) The beaker can hold up to 250 mL. d) The beaker will be the best tool for this lab. 2. Measure the following, circle your estimated digit and inclu ...
Exam 3 Review
... Choose the longest continuous chain of carbon atoms which gives the basic name or stem. Number each carbon atom in the basic chain, starting at the end that gives the lowest number to the first group attached to the main chain (substituent). For each substituent on the chain, we indicate the positio ...
... Choose the longest continuous chain of carbon atoms which gives the basic name or stem. Number each carbon atom in the basic chain, starting at the end that gives the lowest number to the first group attached to the main chain (substituent). For each substituent on the chain, we indicate the positio ...
Chapter 5 Notes
... Unfortunately, thinking of light as waves led to a problem. It was noticed that if light strikes a metal, then sometimes it could cause _____________________ to be emitted (leave the atoms entirely – like in a solar panel); called the _____________________ effect. If light was a wave, then all amoun ...
... Unfortunately, thinking of light as waves led to a problem. It was noticed that if light strikes a metal, then sometimes it could cause _____________________ to be emitted (leave the atoms entirely – like in a solar panel); called the _____________________ effect. If light was a wave, then all amoun ...
Multi-electron atoms
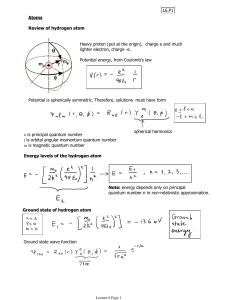
... In H, 3s level is on average 9x further than 1s, so 9*Bohr radius. In Na, 11 protons pull 1s, 2s, 2p closer to nucleus distance of 3s not as far out. Electron in 3s is a bit further than 1s in H, but ~same as 2s in Li. Proximity of electrons in 1s, 2s, 2p is what makes 3s a bit bigger. In case of Na ...
... In H, 3s level is on average 9x further than 1s, so 9*Bohr radius. In Na, 11 protons pull 1s, 2s, 2p closer to nucleus distance of 3s not as far out. Electron in 3s is a bit further than 1s in H, but ~same as 2s in Li. Proximity of electrons in 1s, 2s, 2p is what makes 3s a bit bigger. In case of Na ...
Chemistry Comes Alive: Part A
... • Chemical energy—stored in bonds of chemical substances • Electrical energy—results from movement of charged particles • Mechanical energy—directly involved in moving matter • Radiant or electromagnetic energy—exhibits wavelike properties (i.e., visible light, ultraviolet light, and X-rays) ...
... • Chemical energy—stored in bonds of chemical substances • Electrical energy—results from movement of charged particles • Mechanical energy—directly involved in moving matter • Radiant or electromagnetic energy—exhibits wavelike properties (i.e., visible light, ultraviolet light, and X-rays) ...
quantum numbers - misshoughton.net
... electrons for an atom or ion In fig.2 on p. 187, as atoms become larger & the main energy levels come closer, some sublevels may overlap Generally the sublevels for a particular value of n, increase in energy in the order of s
... electrons for an atom or ion In fig.2 on p. 187, as atoms become larger & the main energy levels come closer, some sublevels may overlap Generally the sublevels for a particular value of n, increase in energy in the order of s
Writing Electron Configuration
... Pauli Exclusion Principle • No two electrons may have the same 4 quantum numbers. • This means: • Maximum of 2 electrons occupy a single atomic orbital • The 2 electrons must have opposite spins, represented by up/down arrows. ...
... Pauli Exclusion Principle • No two electrons may have the same 4 quantum numbers. • This means: • Maximum of 2 electrons occupy a single atomic orbital • The 2 electrons must have opposite spins, represented by up/down arrows. ...
Online Course Evaluation Chapters 15-20
... ¾ The presence of definite energy levels in an atom is true for all atoms. Quantization is characteristic of many quantities in nature ¾ Bohr’s theory worked well for hydrogen and for one-electron ions. But it did not prove as successful for multielectrons. ¾ It is quantum mechanics that finally sol ...
... ¾ The presence of definite energy levels in an atom is true for all atoms. Quantization is characteristic of many quantities in nature ¾ Bohr’s theory worked well for hydrogen and for one-electron ions. But it did not prove as successful for multielectrons. ¾ It is quantum mechanics that finally sol ...
Chapter7 - FSU Chemistry
... 7.96. Electric power is typically stated in units of watts (1W = 1J/s). About 95% of the power output of an incandescent bulb is converted to heat and 5% to light. If 10% of that light shines on your chemistry text, how many photons per second shine on the book from a 75-W bulb? (assume a wavelenght ...
... 7.96. Electric power is typically stated in units of watts (1W = 1J/s). About 95% of the power output of an incandescent bulb is converted to heat and 5% to light. If 10% of that light shines on your chemistry text, how many photons per second shine on the book from a 75-W bulb? (assume a wavelenght ...
Chemistry Midterm Review Study Guide 2012
... Non-metal: Brittle, lack luster, poor conductors of heat and electricity ex. O2, Cl2 Metalloids: Semi conductor’s, solid, have characteristics between metals/ nonmetal, B,Si,Ge,As,Sb,Te 8. What do elements in the same group have in common? The same period? Group or family- vertical, have similar che ...
... Non-metal: Brittle, lack luster, poor conductors of heat and electricity ex. O2, Cl2 Metalloids: Semi conductor’s, solid, have characteristics between metals/ nonmetal, B,Si,Ge,As,Sb,Te 8. What do elements in the same group have in common? The same period? Group or family- vertical, have similar che ...
Exam 1 Topics to Review (McMurry Chpts 1
... 3. Write and interpret isotope symbols for atoms. a. Know atomic number, mass number. b. Find number of protons, neutrons, and electrons from isotope symbol. 4. Isotopes: Calculate atomic mass of an element based on isotopic abundance a. Understand atomic mass unit. ...
... 3. Write and interpret isotope symbols for atoms. a. Know atomic number, mass number. b. Find number of protons, neutrons, and electrons from isotope symbol. 4. Isotopes: Calculate atomic mass of an element based on isotopic abundance a. Understand atomic mass unit. ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.