THERMOCHEMISTRY ENERGETICS/ENTHALPY
... Chemists generally refer to the energy given out when a fuel burns in kJmol-1 because this compares the same number of molecules of each fuel. For use as fuels it is sometimes better to convert the units from kJmol-1 to kJg-1 (OR the energy density) of a fuel ...
... Chemists generally refer to the energy given out when a fuel burns in kJmol-1 because this compares the same number of molecules of each fuel. For use as fuels it is sometimes better to convert the units from kJmol-1 to kJg-1 (OR the energy density) of a fuel ...
Dissociation energy of the C-H bond in chloroform Cl3C
... computational method is essential. We will use density functional theory, specifically the B3LYP functional. Correlated methods work best with large basis sets. We will use the 6-311+G(d,p) basis set that is available in GAMESS under WebMO. The 6-311 basis set has six Gaussian-type functions for cor ...
... computational method is essential. We will use density functional theory, specifically the B3LYP functional. Correlated methods work best with large basis sets. We will use the 6-311+G(d,p) basis set that is available in GAMESS under WebMO. The 6-311 basis set has six Gaussian-type functions for cor ...
Unit B review - mvhs
... In general, as one moves across a row of the periodic table from the alkali metals to the halogens: (A) A, B, and C will decrease. (B) A, B, and C will increase. (C) A will increase, B and C will decrease. (D) A and B will increase, C will decrease. (E) A will decrease, B and C will increase. 15. In ...
... In general, as one moves across a row of the periodic table from the alkali metals to the halogens: (A) A, B, and C will decrease. (B) A, B, and C will increase. (C) A will increase, B and C will decrease. (D) A and B will increase, C will decrease. (E) A will decrease, B and C will increase. 15. In ...
CHEMICAL EQUATIONS NAME PERIOD_______ DATE________
... reaction. In a chemical equation, the substances on the left side of the arrow are the starting substances. These substances are called ______________. The substances on the right side of the arrow are the substances that result from the reaction. These substances are called ____________________. Th ...
... reaction. In a chemical equation, the substances on the left side of the arrow are the starting substances. These substances are called ______________. The substances on the right side of the arrow are the substances that result from the reaction. These substances are called ____________________. Th ...
quantum mechanical model
... Pauli Exclusion Principle: Electrons cannot have the same four quantum numbers within the same atom. Shell: A set of electrons with the same principal quantum number (n). Subshell: A set of electrons with the same azimuthal quantum number (l). ...
... Pauli Exclusion Principle: Electrons cannot have the same four quantum numbers within the same atom. Shell: A set of electrons with the same principal quantum number (n). Subshell: A set of electrons with the same azimuthal quantum number (l). ...
The Periodic Table OL Page 1 of 2 G. Galvin Name: Periodic Table
... -define atomic number (Z) and mass number (A) -define relative atomic mass (Ar) using 12C scale -define isotope -describe the composition of isotopes using hydrogen and carbon as an example -describe the organisation of particles in atoms of elements numbers 1-20 -classify the first twenty elem ...
... -define atomic number (Z) and mass number (A) -define relative atomic mass (Ar) using 12C scale -define isotope -describe the composition of isotopes using hydrogen and carbon as an example -describe the organisation of particles in atoms of elements numbers 1-20 -classify the first twenty elem ...
Quantum Mechanics I Physics 325 Importance of Hydrogen Atom
... atom does not emit energy in the form of electromagnetic radiation – Therefore, the energy of the atom remains constant and classical mechanics can be used to describe the electron’s motion Radiation is emitted by the atom when the electron “jumps” from a more energetic initial state to a lower st ...
... atom does not emit energy in the form of electromagnetic radiation – Therefore, the energy of the atom remains constant and classical mechanics can be used to describe the electron’s motion Radiation is emitted by the atom when the electron “jumps” from a more energetic initial state to a lower st ...
Using mass to calculate molecular formula
... Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number of atoms in the molecule. Percentages of ...
... Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number of atoms in the molecule. Percentages of ...
REACTION DYNAMICS
... What information can infra-red chemiluminescence provide on the energetics and/or dynamics of a chemical reaction? What other experimental methods can be used to obtain similar information? ...
... What information can infra-red chemiluminescence provide on the energetics and/or dynamics of a chemical reaction? What other experimental methods can be used to obtain similar information? ...
Historical burdens on physics 97 The
... lab, gives as a result the work function of the material of the cathode and not that of the anode. The latter would be much greater than the approximately 2 eV which are actually measured. The explanation for this strange behavior is that a small amount of Cesium (we suppose to have a Cesium cathode ...
... lab, gives as a result the work function of the material of the cathode and not that of the anode. The latter would be much greater than the approximately 2 eV which are actually measured. The explanation for this strange behavior is that a small amount of Cesium (we suppose to have a Cesium cathode ...
Bonding
... i. Draw the complete Lewis electron-dot structures for each molecule. ii.In terms of molecular geometry, account for the fact that the CF4 molecule is nonpolar, whereas the SF4 molecule is polar. ...
... i. Draw the complete Lewis electron-dot structures for each molecule. ii.In terms of molecular geometry, account for the fact that the CF4 molecule is nonpolar, whereas the SF4 molecule is polar. ...
7B35.75 Plasma Tubes
... gases, such as argon and neon. A low pressure is necessary so that the gases can be ionized easier, and inert gases must be used so that there is no reaction between the gas and the metal electrode. When the power adapter is connected to the plasma ball, a high voltage, high frequency power supply c ...
... gases, such as argon and neon. A low pressure is necessary so that the gases can be ionized easier, and inert gases must be used so that there is no reaction between the gas and the metal electrode. When the power adapter is connected to the plasma ball, a high voltage, high frequency power supply c ...
Gateway Chemistry Review (Answer Key) Structure and Properties
... Two or more atoms bound so tightly that they behave as a single unit. Linked by covalent bonds Consist of atoms of the same element or different elements Ionic Compound Formed by the attraction of two ions that are oppositely charged. Na+ + Cl- NaCl Practice Identify each of the followin ...
... Two or more atoms bound so tightly that they behave as a single unit. Linked by covalent bonds Consist of atoms of the same element or different elements Ionic Compound Formed by the attraction of two ions that are oppositely charged. Na+ + Cl- NaCl Practice Identify each of the followin ...
5. Atomic models
... The large deflection of alpha particle as seen in the scattering experiment with a thin gold foil must be produced by a close encounter between the alpha particle and a very small but massive kernel inside the atom (c.f. a diffused distribution of the positive charge as assumed in plum-pudding model ...
... The large deflection of alpha particle as seen in the scattering experiment with a thin gold foil must be produced by a close encounter between the alpha particle and a very small but massive kernel inside the atom (c.f. a diffused distribution of the positive charge as assumed in plum-pudding model ...
Electronic Absorption Spectroscopy
... molecule. Then, for oriented samples, the N-H stretch and amide I bands should preferentially absorb IR light when the polarization is parallel to the helix axis. The amide I1 band should have orthogonal polarization. In an actual protein or polypeptide the situation is more complex because of inter ...
... molecule. Then, for oriented samples, the N-H stretch and amide I bands should preferentially absorb IR light when the polarization is parallel to the helix axis. The amide I1 band should have orthogonal polarization. In an actual protein or polypeptide the situation is more complex because of inter ...
Chemistry 101 Chapter 4 Elements, Atoms, and Ions = =
... elements are diatomic and they consist of two atoms. The atoms of these elements have special affinities for each other and they are connected to each other by a chemical bond (for example, N2 and O2). Some elements are polyatomic and they consist of many atoms (for example, O3 and S8). Allotropes: ...
... elements are diatomic and they consist of two atoms. The atoms of these elements have special affinities for each other and they are connected to each other by a chemical bond (for example, N2 and O2). Some elements are polyatomic and they consist of many atoms (for example, O3 and S8). Allotropes: ...
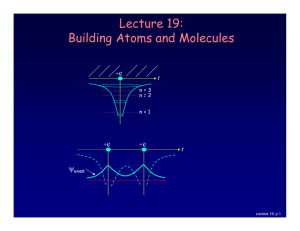
Lecture 19: Building Atoms and Molecules
... Magnetic resonance imaging (MRI) depends on the absorption of electromagnetic radiation by the nuclear spin of the hydrogen atoms in our bodies. The nucleus is a proton with spin ½, so in a magnetic field B there are two energy states. The proton’s magnetic moment is µp = 1.41 x 10-26 J /Tesla. ...
... Magnetic resonance imaging (MRI) depends on the absorption of electromagnetic radiation by the nuclear spin of the hydrogen atoms in our bodies. The nucleus is a proton with spin ½, so in a magnetic field B there are two energy states. The proton’s magnetic moment is µp = 1.41 x 10-26 J /Tesla. ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.