Chapter 14 – Chemical Reactions
... Reactants – the _____________ materials of a chemical _____________ Products – the substances _____________ as a _____________ of a chemical _____________ Coefficient – a _____________ placed in _____________ of a chemical _____________ or _____________ All chemical equations must be balanced. Steps ...
... Reactants – the _____________ materials of a chemical _____________ Products – the substances _____________ as a _____________ of a chemical _____________ Coefficient – a _____________ placed in _____________ of a chemical _____________ or _____________ All chemical equations must be balanced. Steps ...
PHY492: Nuclear & Particle Physics Lecture 4 Nature of the nuclear force
... •angular momentum (spin) •symmetries (parity) •magnetic moments ...
... •angular momentum (spin) •symmetries (parity) •magnetic moments ...
Quantum Mechanics
... Explanation of Classical “Problems” The effect is not observed below a certain cutoff frequency since the photon energy must be greater than or equal to the work function – Without this, electrons are not emitted, regardless of the intensity of the light The maximum KE depends only on the frequ ...
... Explanation of Classical “Problems” The effect is not observed below a certain cutoff frequency since the photon energy must be greater than or equal to the work function – Without this, electrons are not emitted, regardless of the intensity of the light The maximum KE depends only on the frequ ...
Chapter 7
... • Calculate the wavelength of an electron traveling with a speed of 2.65x106 m/s • Pay close attention to your units!!! (remember that 1 J = 1 kg*m2/s2 ...
... • Calculate the wavelength of an electron traveling with a speed of 2.65x106 m/s • Pay close attention to your units!!! (remember that 1 J = 1 kg*m2/s2 ...
QUANTUM THEORY
... C) Photons travel at the speed of light in a vacuum. D) Photons have been brought to rest by applying a strong magnetic field to them. E) The energy of a photon is proportional to its frequency. The Photoelectric Effect 17. Photons of what minimum frequency are required to remove electrons from gold ...
... C) Photons travel at the speed of light in a vacuum. D) Photons have been brought to rest by applying a strong magnetic field to them. E) The energy of a photon is proportional to its frequency. The Photoelectric Effect 17. Photons of what minimum frequency are required to remove electrons from gold ...
C. - Knights of The Periodic Table
... equal numbers of atoms of each type on both sides of the equation. This illustrates the principle of — A. conservation of energy B. conservation of mass C. action and reaction D. natural selection ...
... equal numbers of atoms of each type on both sides of the equation. This illustrates the principle of — A. conservation of energy B. conservation of mass C. action and reaction D. natural selection ...
Nuclear Physics
... 1. Add up the mass (in atomic mass units, u) of the reactants. 2. Add up the mass (in atomic mass units, u) of the products. 3. Find the difference between reactant and product mass. The missing mass has been converted to energy. 4. Convert mass to kg ( 1 u = 1.66 x 10-27 kg) 5. Use E = mc2 to calcu ...
... 1. Add up the mass (in atomic mass units, u) of the reactants. 2. Add up the mass (in atomic mass units, u) of the products. 3. Find the difference between reactant and product mass. The missing mass has been converted to energy. 4. Convert mass to kg ( 1 u = 1.66 x 10-27 kg) 5. Use E = mc2 to calcu ...
Lecture 2
... • Allowed electron energy levels in an atom give rise to bands of allowed electron energy levels in a crystal. – The valence band is the highest nearly-filled band. – The conduction band is the lowest nearly-empty band. ...
... • Allowed electron energy levels in an atom give rise to bands of allowed electron energy levels in a crystal. – The valence band is the highest nearly-filled band. – The conduction band is the lowest nearly-empty band. ...
hydrogen
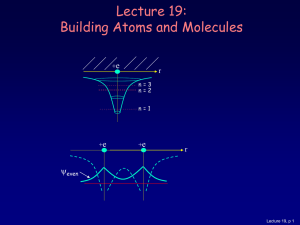
... The three dimensional behavior of the probability density is completely dependent on the product of the radial probability density Pnl ( r ) Rnl* ( r ) Rnl ( r ) and a directionally dependent modulation factor *lml ( )lml ( ) . The probability of finding the electron at any position within a s ...
... The three dimensional behavior of the probability density is completely dependent on the product of the radial probability density Pnl ( r ) Rnl* ( r ) Rnl ( r ) and a directionally dependent modulation factor *lml ( )lml ( ) . The probability of finding the electron at any position within a s ...
Electronic Structure
... it emits a quantum of energy E in radiation of definite frequency, . Since E for the change from E2 to E1 is always the same in a given atom, and h is a constant , must be a constant. Therefore, radiation always has the same energy and is always of the same frequency for this particular electro ...
... it emits a quantum of energy E in radiation of definite frequency, . Since E for the change from E2 to E1 is always the same in a given atom, and h is a constant , must be a constant. Therefore, radiation always has the same energy and is always of the same frequency for this particular electro ...
Name: Date: Period: _____ Unit 2 Notes, Part 1 – The Basics of
... 2. Atoms are the smallest unit of matter. Each different type of atom represents an element (ex: hydrogen, oxygen, carbon). Scientists have created a chart called the periodic table of elements to organize elements by their atomic properties. 3. Four elements—carbon (C), oxygen (O), hydrogen (H), an ...
... 2. Atoms are the smallest unit of matter. Each different type of atom represents an element (ex: hydrogen, oxygen, carbon). Scientists have created a chart called the periodic table of elements to organize elements by their atomic properties. 3. Four elements—carbon (C), oxygen (O), hydrogen (H), an ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.