![Neutral ionic liquid [BMIm]BF4 promoted highly selective](http://s1.studyres.com/store/data/017897985_1-047f9869d5604c115b21339541ccfffe-300x300.png)
Neutral ionic liquid [BMIm]BF4 promoted highly selective
... Our previous investigations on the conversion of tert-butanol revealed that the reactivity of tert-butanol could be greatly improved by the use of tetrafluoroborate ionic liquids as media [26,27]. Therefore, we consequently investigated the esterification reaction of tert-butanol in [BMIm]BF4 , whic ...
... Our previous investigations on the conversion of tert-butanol revealed that the reactivity of tert-butanol could be greatly improved by the use of tetrafluoroborate ionic liquids as media [26,27]. Therefore, we consequently investigated the esterification reaction of tert-butanol in [BMIm]BF4 , whic ...
chemistry intermediate may 2010 marking scheme
... (a) A is a white crystalline solid with a high melting point. A gives a lilac colour when flametested. When aqueous silver nitrate is added to a solution of A, a white precipitate B, insoluble in dilute nitric acid is obtained. ...
... (a) A is a white crystalline solid with a high melting point. A gives a lilac colour when flametested. When aqueous silver nitrate is added to a solution of A, a white precipitate B, insoluble in dilute nitric acid is obtained. ...
1984 Advanced Placement Exam
... boiling points given above. The relatively high likely formed with magnesium, Mg, is boiling point of HF can be correctly explained (A) MgX (C) MgX2 (E) Mg3X2 by which of the following? (B) Mg2X (D) MgX3 (A) HF gas is more ideal. (B) HF is the strongest acid. (C) HF molecules have a smaller dipole 2 ...
... boiling points given above. The relatively high likely formed with magnesium, Mg, is boiling point of HF can be correctly explained (A) MgX (C) MgX2 (E) Mg3X2 by which of the following? (B) Mg2X (D) MgX3 (A) HF gas is more ideal. (B) HF is the strongest acid. (C) HF molecules have a smaller dipole 2 ...
Pdf - Text of NPTEL IIT Video Lectures
... we all know that this nitrogen and oxygen can provide nicely the corresponding porbitals for interaction to the metal centre. But, previously what we are seen that these electrons present in this p-orbitals of the different ligands are providing the corresponding crystal field environment along the ...
... we all know that this nitrogen and oxygen can provide nicely the corresponding porbitals for interaction to the metal centre. But, previously what we are seen that these electrons present in this p-orbitals of the different ligands are providing the corresponding crystal field environment along the ...
Chapter One
... again and again and again. In theory, we should eventually end up witl1 a single gold atom. If we tried to split this atom in half, we would end up w ith something that no longer retains any of the characteristics of the element. An atom is there fore the smallest particle that can be used to ident ...
... again and again and again. In theory, we should eventually end up witl1 a single gold atom. If we tried to split this atom in half, we would end up w ith something that no longer retains any of the characteristics of the element. An atom is there fore the smallest particle that can be used to ident ...
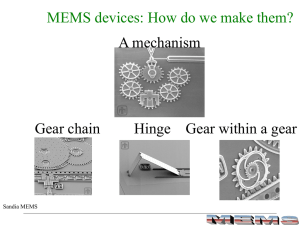
MEMS Processing
... - Amorphous/columnar grained structures: Compressive stress - Equiaxed grained structures: Tensile stress - Thick films have less stress than thinner films -ANNEALING CAN REDUCE STRESSES BY A FACTOR OF 10-100 ...
... - Amorphous/columnar grained structures: Compressive stress - Equiaxed grained structures: Tensile stress - Thick films have less stress than thinner films -ANNEALING CAN REDUCE STRESSES BY A FACTOR OF 10-100 ...
STUDY GUIDE
... multiple bonds, and they are more reactive than alkanes. They may participate in addition reactions, including hydrogenation, halogenation, hydrohalogenation, and hydration. A naming scheme has been established by the International Union of Pure and Applied Chemistry (IUPAC) to name the organic comp ...
... multiple bonds, and they are more reactive than alkanes. They may participate in addition reactions, including hydrogenation, halogenation, hydrohalogenation, and hydration. A naming scheme has been established by the International Union of Pure and Applied Chemistry (IUPAC) to name the organic comp ...
Chemistry Curriculum Map - Belle Vernon Area School District
... Diagnostic – Pretest on “The Periodic Table” (Given before Chapter 4), Pretest on “Bond Types” (Given before Chapter 5), Pretest on “Common Compounds” and Naming (Given before Chapter 5). Each posttest given after end of chapter. Benchmark – Study Island: Systems, Models, and Patterns (given During ...
... Diagnostic – Pretest on “The Periodic Table” (Given before Chapter 4), Pretest on “Bond Types” (Given before Chapter 5), Pretest on “Common Compounds” and Naming (Given before Chapter 5). Each posttest given after end of chapter. Benchmark – Study Island: Systems, Models, and Patterns (given During ...
Chapter 3 Stoichiometry: Calculations with Chemical
... (d).None of the above Explanation: Convert the number of atoms to number of moles and then using the molar mass of magnesium, calculate the number of grams. As the number of atoms is being converted to grams the answer will be a small ...
... (d).None of the above Explanation: Convert the number of atoms to number of moles and then using the molar mass of magnesium, calculate the number of grams. As the number of atoms is being converted to grams the answer will be a small ...
Topic 4 - Lloyd Crosby
... c. A complex ion is an ion in which a ligand is covalently bound to a metal. d. A ligand is any molecule or ion connected to the central ion or atom of a complex by means of a coordinate covalent bond. e. Coordination number The coordination number is the total number of bonds the metal ion forms wi ...
... c. A complex ion is an ion in which a ligand is covalently bound to a metal. d. A ligand is any molecule or ion connected to the central ion or atom of a complex by means of a coordinate covalent bond. e. Coordination number The coordination number is the total number of bonds the metal ion forms wi ...
Module 29: General Chemistry Instructor Guide – Answer Key
... Ans: A physical change in matter is a change in the form of matter but not in its chemical identity. A chemical change in matter is a change in which one or more kinds of matter transform into a new kind of matter. ...
... Ans: A physical change in matter is a change in the form of matter but not in its chemical identity. A chemical change in matter is a change in which one or more kinds of matter transform into a new kind of matter. ...
Section 3.5 Ionic Compounds: Formulas and Names
... Molecular Compounds: Formulas and Names Solution • The compound NCl3 is nitrogen trichloride , but AlCl3 is just aluminum chloride. Why? • NCl3 is a covalent (molecular compound). Since nitrogen and chlorine can combine more than one way it is necessary to indicate the number of chlorines. • AlCl3 i ...
... Molecular Compounds: Formulas and Names Solution • The compound NCl3 is nitrogen trichloride , but AlCl3 is just aluminum chloride. Why? • NCl3 is a covalent (molecular compound). Since nitrogen and chlorine can combine more than one way it is necessary to indicate the number of chlorines. • AlCl3 i ...
C7 Revision Notes 2015
... •Sports men and women have to provide urine samples to check they have not been taking drugs this is done in front of a testing officer to ensure it is not tampered with and labelled with a unique code so the lab does not know the identity of the ...
... •Sports men and women have to provide urine samples to check they have not been taking drugs this is done in front of a testing officer to ensure it is not tampered with and labelled with a unique code so the lab does not know the identity of the ...
Redox
... Before metallurgy, humans discovered fire. The technology of fire has been crucial in the development of human cultures, but only relatively recently (18th century) have we come to realize the role of oxygen in burning. Understanding the connection of corrosion (rusting, tarnishing, etc.) and burnin ...
... Before metallurgy, humans discovered fire. The technology of fire has been crucial in the development of human cultures, but only relatively recently (18th century) have we come to realize the role of oxygen in burning. Understanding the connection of corrosion (rusting, tarnishing, etc.) and burnin ...
Chapter 1: Matter and Measurement
... Read atomic masses. Read the ions formed by main group elements. Read the electron configuration. Learn trends in physical and chemical properties. ...
... Read atomic masses. Read the ions formed by main group elements. Read the electron configuration. Learn trends in physical and chemical properties. ...
Chem 2A Final Review
... 60. The oxidation number (oxidation state) of sulfur in the following are? K2SO2, K2S2O3, K2S 61. According to Le Chatelier’s principle what effects will take place on the equilibrium of the following reaction: CO2 + H2 H2O + CO a) Increase [H2] b) Increase [H2O] c) remove H2O and CO ...
... 60. The oxidation number (oxidation state) of sulfur in the following are? K2SO2, K2S2O3, K2S 61. According to Le Chatelier’s principle what effects will take place on the equilibrium of the following reaction: CO2 + H2 H2O + CO a) Increase [H2] b) Increase [H2O] c) remove H2O and CO ...
CHEMISTRY – Summer Assignment Solutions 2013
... In the human body, the toxic compound hydrogen cyanide is neutralized by the acid, H 2S2O3, according to the reaction: HCN + H2S2O3 HCNS + H2SO3. If 1.000 mg of H2S2O3, is available in the body, will this be enough to neutralize 2.000 mg of HCN swallowed by a person? [hint – focus on the mole rati ...
... In the human body, the toxic compound hydrogen cyanide is neutralized by the acid, H 2S2O3, according to the reaction: HCN + H2S2O3 HCNS + H2SO3. If 1.000 mg of H2S2O3, is available in the body, will this be enough to neutralize 2.000 mg of HCN swallowed by a person? [hint – focus on the mole rati ...
formula
... They go through several examples of the types of problems I have assigned. If you cannot find my webpage, email me and I will send you the link. •AP Chemistry Boot Camp: AP Chemistry Boot Camp will run July 14th-17th. You are highly encouraged to sign up. We will mostly be covering Units 3 and 4 dur ...
... They go through several examples of the types of problems I have assigned. If you cannot find my webpage, email me and I will send you the link. •AP Chemistry Boot Camp: AP Chemistry Boot Camp will run July 14th-17th. You are highly encouraged to sign up. We will mostly be covering Units 3 and 4 dur ...
Use the following answers for questions 10
... (B) It depends on the intermolecular forces of attraction between molecules of X, Y, and Z. (C) It depends on the relative molecular masses of X, Y, and Z. (D) It depends on the average distance traveled between molecular collisions. (E) It can be calculated with knowledge only of the volume of the ...
... (B) It depends on the intermolecular forces of attraction between molecules of X, Y, and Z. (C) It depends on the relative molecular masses of X, Y, and Z. (D) It depends on the average distance traveled between molecular collisions. (E) It can be calculated with knowledge only of the volume of the ...
in Peptide Synthesis, Molecular Recognition
... peptide or protein backbones hampers this strategy from becoming a routinely applied tool in biomimetic chemistry. During our ongoing work on peptidomimetics for tuning secondary structure formation, we have noticed that the temporary transformation of serine (Ser), threonine (Thr) and cysteine (Cys ...
... peptide or protein backbones hampers this strategy from becoming a routinely applied tool in biomimetic chemistry. During our ongoing work on peptidomimetics for tuning secondary structure formation, we have noticed that the temporary transformation of serine (Ser), threonine (Thr) and cysteine (Cys ...
Class 11 Class 12 The p- Block Element • Group13 (B to Tl
... Behavior in Aqueous Solutions 1 Al, Ga, In and Tl exhibit a well-defined aqueous chemistry in their tripositive states. Species like [M(OH)4 ] -, [M(H 2 O)2(OH)4 ] -, [M(OH2)6]3+ for M = Al, Ga, In, exist in aqueous solution. 2. Al, Ga. In and T1 ions exist as octahedral aqua ions, [M(OH2)6] 3 + ...
... Behavior in Aqueous Solutions 1 Al, Ga, In and Tl exhibit a well-defined aqueous chemistry in their tripositive states. Species like [M(OH)4 ] -, [M(H 2 O)2(OH)4 ] -, [M(OH2)6]3+ for M = Al, Ga, In, exist in aqueous solution. 2. Al, Ga. In and T1 ions exist as octahedral aqua ions, [M(OH2)6] 3 + ...
Bonding in Low-Coordinate Complexes - Munin
... across structural to catalytic. Transition metal ions have a rich chemistry due to close-lying energy bands made up of partly filled d-orbitals, and thus serve as unique agents in a variety of biological processes. In particular, this is the case for the middle and late first-row transition metal io ...
... across structural to catalytic. Transition metal ions have a rich chemistry due to close-lying energy bands made up of partly filled d-orbitals, and thus serve as unique agents in a variety of biological processes. In particular, this is the case for the middle and late first-row transition metal io ...
Introduction to Inorganic Chemistry
... order to achieve its ends. This means that a good chemist is one who not only has a mastery of chemical theory, but also a good knowledge of chemical facts. With such a knowledge, he can direct a trial and error approach to practical problems in the most promising directions. Inorganic Chemistry Org ...
... order to achieve its ends. This means that a good chemist is one who not only has a mastery of chemical theory, but also a good knowledge of chemical facts. With such a knowledge, he can direct a trial and error approach to practical problems in the most promising directions. Inorganic Chemistry Org ...