Slides
... E : edge (bond) set Canonical representation of subgraph surrounding a particular atom include atoms and bonds up to a predefined distance (height) ...
... E : edge (bond) set Canonical representation of subgraph surrounding a particular atom include atoms and bonds up to a predefined distance (height) ...
Review - Discount Flies
... Size: Increases going down a group, decreases going left to right Ionization Energy – the energy required to remove an electron from an atom. decreases going down a group, increases going left to right across a period. ...
... Size: Increases going down a group, decreases going left to right Ionization Energy – the energy required to remove an electron from an atom. decreases going down a group, increases going left to right across a period. ...
OXIDATION NUMBERS
... • CO2 is neutral, so the sum of the oxidation numbers must be zero • one element must have a positive ON, the other must be negative • the more electronegative species will have the negative value • electronegativity increases across a period and decreases down a group • O is further to the right in ...
... • CO2 is neutral, so the sum of the oxidation numbers must be zero • one element must have a positive ON, the other must be negative • the more electronegative species will have the negative value • electronegativity increases across a period and decreases down a group • O is further to the right in ...
Starter S-30
... -reactants can be impure -reactions may not go to completion -may compete with smaller “side” reactions In some reactions as little as 60% yield is considered a good result ...
... -reactants can be impure -reactions may not go to completion -may compete with smaller “side” reactions In some reactions as little as 60% yield is considered a good result ...
Chemistry - talcher autonomous college
... application, Solvation energy. (ii) Covalent bond: Lewis structure, Valence Bond theory (Heitler-London approach). Energetics of hybridization, equivalent and non-equivalent hybrid orbitals. Bent’s rule, Resonance and resonance energy, Molecular orbital theory. Molecular orbital diagrams of diatomic ...
... application, Solvation energy. (ii) Covalent bond: Lewis structure, Valence Bond theory (Heitler-London approach). Energetics of hybridization, equivalent and non-equivalent hybrid orbitals. Bent’s rule, Resonance and resonance energy, Molecular orbital theory. Molecular orbital diagrams of diatomic ...
chemistry - billpalmer
... 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
... 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
Chapter 4 2013
... Water is a polar molecule due to oxygen’s ability to attract electrons (called electronegativity) more so than hydrogen. e- ...
... Water is a polar molecule due to oxygen’s ability to attract electrons (called electronegativity) more so than hydrogen. e- ...
Atoms, Molecules, and Ions
... proportions or the law of constant composition. The suggestion that the numbers of atoms of the elements in a given compound always exist in the same ratio is consistent with these observations. For example, when different samples of isooctane (a component of gasoline and one of the standards used i ...
... proportions or the law of constant composition. The suggestion that the numbers of atoms of the elements in a given compound always exist in the same ratio is consistent with these observations. For example, when different samples of isooctane (a component of gasoline and one of the standards used i ...
Detailed characterization of anodic bonding process between glass
... cleaning method ŽH 2 SO4 –H 2 O 2 and HF.. The generic bonding time versus applied voltage curves for the two cleaning methods are compared in Fig. 5. It can be seen that H 2 SO4 –H 2 O 2 and HF cleaning provides better bonding performance than acetone cleaning. At an applied voltage of 500 V, the b ...
... cleaning method ŽH 2 SO4 –H 2 O 2 and HF.. The generic bonding time versus applied voltage curves for the two cleaning methods are compared in Fig. 5. It can be seen that H 2 SO4 –H 2 O 2 and HF cleaning provides better bonding performance than acetone cleaning. At an applied voltage of 500 V, the b ...
Dissociation
... — Learn to use your reference tables — it’s fun and if you take advantage of this special limited time offer, it’s absolutely free — The guidelines are useful in helping to predict what will happen if the solutions of two different soluble compounds are mixed — If the mixing results in a combination ...
... — Learn to use your reference tables — it’s fun and if you take advantage of this special limited time offer, it’s absolutely free — The guidelines are useful in helping to predict what will happen if the solutions of two different soluble compounds are mixed — If the mixing results in a combination ...
Module 9 Methods for Structure Determination Lecture 24 UV
... Under electron bombardment, methane loses a bonding electron to give CH4+• which reacts with an unionized methane molecule to give CH3• and CH5+. This unstable compound (CH5+) is a powerful acid, and can protonate just about any other molecule. When it protonates our sample, a proton has been added ...
... Under electron bombardment, methane loses a bonding electron to give CH4+• which reacts with an unionized methane molecule to give CH3• and CH5+. This unstable compound (CH5+) is a powerful acid, and can protonate just about any other molecule. When it protonates our sample, a proton has been added ...
PHYSICAL SETTING CHEMISTRY
... word or expression that, of those given, best completes the statement or answers the question. Some questions may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry. 6 The atomic mass of magnesium is the weighted average of the atomic masses of (1) all of the artific ...
... word or expression that, of those given, best completes the statement or answers the question. Some questions may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry. 6 The atomic mass of magnesium is the weighted average of the atomic masses of (1) all of the artific ...
Oxidation numbers
... MnO4Using rule 5, O has an O.N. of –2 Using rule 4, the O.N. of the elements must add up to -1 Mn must have an O.N. of +7 in order to cancel out 4 x –2 = -8 of the O’s +7 -8 = -1 (the charge on the ion) The ion is the Manganate (VII) ion ...
... MnO4Using rule 5, O has an O.N. of –2 Using rule 4, the O.N. of the elements must add up to -1 Mn must have an O.N. of +7 in order to cancel out 4 x –2 = -8 of the O’s +7 -8 = -1 (the charge on the ion) The ion is the Manganate (VII) ion ...
Symbol Protons Neutons Electrons Name
... sharing some of their valence electrons. Seven nonmetal elements are most commonly found in nature as diatomic molecules. • When two or more different nonmetals combine, a molecular compound is formed. Department of Chemistry and Biochemistry ...
... sharing some of their valence electrons. Seven nonmetal elements are most commonly found in nature as diatomic molecules. • When two or more different nonmetals combine, a molecular compound is formed. Department of Chemistry and Biochemistry ...
Chemistry - Sanskriti School
... Unit X : s-Block Elements (Alkali and Alkaline earth metals) Group 1 and Group 2 elements: General introduction, electronic configuration, occurrence, anomalous properties of the first element of each group, diagonal relationship, trends in the variation of properties (such as ionization enthalpy, a ...
... Unit X : s-Block Elements (Alkali and Alkaline earth metals) Group 1 and Group 2 elements: General introduction, electronic configuration, occurrence, anomalous properties of the first element of each group, diagonal relationship, trends in the variation of properties (such as ionization enthalpy, a ...
Elements – (Metals)
... Electrons are excited to higher energy state by light all wavelengths. Electrons fall back to lower levels and re-emit light so metals have shiny surface. 3) Deform under stress without cleaving Held together by mobile electrons Ductile – drawn into wire Malleable – pounded into plate 4) Form positi ...
... Electrons are excited to higher energy state by light all wavelengths. Electrons fall back to lower levels and re-emit light so metals have shiny surface. 3) Deform under stress without cleaving Held together by mobile electrons Ductile – drawn into wire Malleable – pounded into plate 4) Form positi ...
Study Material - Class- XI- Chemistry
... Precision refers to the closeness of various measurements for the same quantity. Accuracy is the agreement of a particular value to the true value of the result. Significant Figures The reliability of a measurement is indicated by the number of digits used to represent it. To express it more accurat ...
... Precision refers to the closeness of various measurements for the same quantity. Accuracy is the agreement of a particular value to the true value of the result. Significant Figures The reliability of a measurement is indicated by the number of digits used to represent it. To express it more accurat ...
E:\My Documents\sch4u\SCH4U review McKay answers.wpd
... between their boiling points. Which of these two substances would have the higher boiling point? Explain your answer. CH3F, has a higher boiling point because it is polar and has greater London forces. 3) The element iodine exists as solid crystals composed of I2 molecules. A chemist wishing to diss ...
... between their boiling points. Which of these two substances would have the higher boiling point? Explain your answer. CH3F, has a higher boiling point because it is polar and has greater London forces. 3) The element iodine exists as solid crystals composed of I2 molecules. A chemist wishing to diss ...
MC84 - Southchemistry.com
... (B) It depends on the intermolecular forces of attraction between molecules of X, Y, and Z. (C) It depends on the relative molecular masses of X, Y, and Z. (D) It depends on the average distance traveled between molecular collisions. (E) It can be calculated with knowledge only of the volume of the ...
... (B) It depends on the intermolecular forces of attraction between molecules of X, Y, and Z. (C) It depends on the relative molecular masses of X, Y, and Z. (D) It depends on the average distance traveled between molecular collisions. (E) It can be calculated with knowledge only of the volume of the ...
Chapter 4: Chemical Reaction Dynamics
... 4.4.4 Reaction mechanisms from angular scattering The angular distribution of scattering products reflecting the differential scattering cross section can be measured in crossed molecular beam experiments. The angular distribution of the scattering products is measured with a moveable detector in t ...
... 4.4.4 Reaction mechanisms from angular scattering The angular distribution of scattering products reflecting the differential scattering cross section can be measured in crossed molecular beam experiments. The angular distribution of the scattering products is measured with a moveable detector in t ...
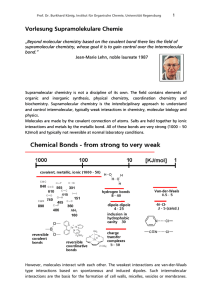
Vorlesung Supramolekulare Chemie
... Ag+ form complexes with π-systems. These interactions are strong and are not considered non-covalent, because of the bonding situation involving the π-orbital of the unsaturated ligand and the d-orbitals of the metal ion. However, alkali- and earth alkali metal cations show much weaker interactions ...
... Ag+ form complexes with π-systems. These interactions are strong and are not considered non-covalent, because of the bonding situation involving the π-orbital of the unsaturated ligand and the d-orbitals of the metal ion. However, alkali- and earth alkali metal cations show much weaker interactions ...