The Covalent Bond and Carbon
... These mathematical expressions are called wave equations since they are based upon the concept that e-’s show properties not only of particles but also of electromagnetic waves. These wave equations have a series of solutions called wave functions which allow us to predict the volume of space around ...
... These mathematical expressions are called wave equations since they are based upon the concept that e-’s show properties not only of particles but also of electromagnetic waves. These wave equations have a series of solutions called wave functions which allow us to predict the volume of space around ...
Spring Benchmark Exam
... 30. A technician prepared a solution by heating 100 milliliters of distilled water while adding KCl crystals until no more KCl would dissolve. She then capped the clear solution and set it aside on the lab bench. After several hours she noticed the solution had become cloudy and some solid had settl ...
... 30. A technician prepared a solution by heating 100 milliliters of distilled water while adding KCl crystals until no more KCl would dissolve. She then capped the clear solution and set it aside on the lab bench. After several hours she noticed the solution had become cloudy and some solid had settl ...
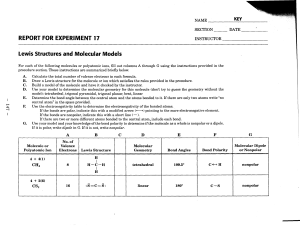
Lewis Structures and Molecular Models - Corwith-Wesley
... procedure section. These instructions are summarized briefly below. ...
... procedure section. These instructions are summarized briefly below. ...
According to Democritus, atoms were small
... This is the Greek philosopher Democritus who began the search for a description of matter more than 2400 years ago. He asked: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times a piece of matter could be divided? His theory: Matter could NOT ...
... This is the Greek philosopher Democritus who began the search for a description of matter more than 2400 years ago. He asked: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times a piece of matter could be divided? His theory: Matter could NOT ...
Zn + HCl → ZnCl 2 + H2 NaOH + H3PO4 → Na3PO4 + H2O N2 +
... Look at only one type of atom at a time. Start with atoms that appear only once on each side of the equation. Once those are balanced, try to balance atoms that appear in more than one species on either side of the reaction. Tip: If there is a molecule with only one type of atom (eg, O2), so ...
... Look at only one type of atom at a time. Start with atoms that appear only once on each side of the equation. Once those are balanced, try to balance atoms that appear in more than one species on either side of the reaction. Tip: If there is a molecule with only one type of atom (eg, O2), so ...
Chapter 4: Introduction to Earth Chemistry Section 1 Notes
... of matter that can exist by itself and retain all of a substance’s chemical properties Chemical Formulas A chemical formula is a _________________________________________________________________________________ ______________ and the _________________________________________ that are required to mak ...
... of matter that can exist by itself and retain all of a substance’s chemical properties Chemical Formulas A chemical formula is a _________________________________________________________________________________ ______________ and the _________________________________________ that are required to mak ...
200 ways to pass the regents
... 83. The elements in Group 1 are the alkali metals. 84. The elements in Group 2 are the alkaline earth metals. 85. The elements in Group 17 are the halogens. 86. The elements in Group 18 are the noble gases. 87. Use Table S to compare and look up the properties of specific elements. 88. Energy is rel ...
... 83. The elements in Group 1 are the alkali metals. 84. The elements in Group 2 are the alkaline earth metals. 85. The elements in Group 17 are the halogens. 86. The elements in Group 18 are the noble gases. 87. Use Table S to compare and look up the properties of specific elements. 88. Energy is rel ...
Chapter 2 Atoms, Molecules and Ions
... Chemical Formula: In which the symbols for the elements are used to indicate the types of atoms present and subscripts are used to indicate the relative numbers of atoms. CO2 indicates each molecule contains 1 atom of carbon and 2 atoms of oxygen. Structural Formula: In which the individual bonds ar ...
... Chemical Formula: In which the symbols for the elements are used to indicate the types of atoms present and subscripts are used to indicate the relative numbers of atoms. CO2 indicates each molecule contains 1 atom of carbon and 2 atoms of oxygen. Structural Formula: In which the individual bonds ar ...
Unit 16 Worksheet - Jensen Chemistry
... 15. Lithium, sodium, potassium, and rubidium are all members of the a. alkali metals ...
... 15. Lithium, sodium, potassium, and rubidium are all members of the a. alkali metals ...
Note-taking Strategy Your notes should contain a title with
... was the tiniest particle of matter. He thought of it as a tiny, indivisible, indestructible particle. However, his ideas were not based on any scientific experimenting. 2000 years later in England, John Dalton did a bunch of experiments and came up with a revised atomic theory. He agreed that the ...
... was the tiniest particle of matter. He thought of it as a tiny, indivisible, indestructible particle. However, his ideas were not based on any scientific experimenting. 2000 years later in England, John Dalton did a bunch of experiments and came up with a revised atomic theory. He agreed that the ...
200 Ways to Pass the Chemistry - Home 15-16
... Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl 95. Covalent bonds non-metal with non-metal form when two atoms share a pair of electrons. How many covalent bonds are found in a nitrogen (N2) molecule? 96. Ionic bonds metal with non-metal form when o ...
... Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl 95. Covalent bonds non-metal with non-metal form when two atoms share a pair of electrons. How many covalent bonds are found in a nitrogen (N2) molecule? 96. Ionic bonds metal with non-metal form when o ...
Chemistry 1. The Periodic Table displays the
... how to solve problems involving heat flow and temperature changes, using known values of specific heat, and latent heat of phase change. apply Hess’s Law to calculate enthalpy change in a reaction. the Gibbs free energy equation explain why a reaction would be spontaneous. ...
... how to solve problems involving heat flow and temperature changes, using known values of specific heat, and latent heat of phase change. apply Hess’s Law to calculate enthalpy change in a reaction. the Gibbs free energy equation explain why a reaction would be spontaneous. ...
INFLUENCE OF A DEFORMATION OF NH3 MOLECULE ON A
... Additional information about the force matrix of an ammonia molecule within the framework of studying the influence of molecule’s deformation on the chemical bond deviation is obtained. The elastic constants of the central forces between chemically unbound atoms of hydrogen, which are usually neglec ...
... Additional information about the force matrix of an ammonia molecule within the framework of studying the influence of molecule’s deformation on the chemical bond deviation is obtained. The elastic constants of the central forces between chemically unbound atoms of hydrogen, which are usually neglec ...
solution is a solution that contains the maximum amount of solute
... Instructions: The midterm consists of two sections (A, 36 points; B, 64 points). Answer each question in the space provided. Write your name at the top of the test in the space provided. Read each question carefully. There is a periodic table provided on the last page of the test. Including the peri ...
... Instructions: The midterm consists of two sections (A, 36 points; B, 64 points). Answer each question in the space provided. Write your name at the top of the test in the space provided. Read each question carefully. There is a periodic table provided on the last page of the test. Including the peri ...
Document
... The concept of temperature and changes of phase between solid, liquid, and gas are traditionally considered part of chemistry, as are the gas laws. These kinds of changes in matter are called physical changes, because matter changes physical form but one substance does not change into a complete ...
... The concept of temperature and changes of phase between solid, liquid, and gas are traditionally considered part of chemistry, as are the gas laws. These kinds of changes in matter are called physical changes, because matter changes physical form but one substance does not change into a complete ...
sch3u unit 1 test: matter
... 10. Copper (II) hydroxide is composed of a. 2 elements, 2 atoms b. 2 elements, 3 atoms c. 3 elements, 4 atoms d. 3 elements, 5 atoms ...
... 10. Copper (II) hydroxide is composed of a. 2 elements, 2 atoms b. 2 elements, 3 atoms c. 3 elements, 4 atoms d. 3 elements, 5 atoms ...
Atoms, Ions and Molecules
... It is the number of protons that determines which element an atom belongs to. Hydrogen is the simplest atom with only one proton and one electron, this is why it is the most abundant element in ...
... It is the number of protons that determines which element an atom belongs to. Hydrogen is the simplest atom with only one proton and one electron, this is why it is the most abundant element in ...
Unit 1 Review, pages 138–145
... repeating pattern by strong electrostatic forces. (b) Ionic compounds are hard because their strong bonds resist being stretched. (c) An ionic compound breaks when struck with a hammer because its lattice structure is offset, and like charges repel each other. (d) Ionic compounds conduct electricity ...
... repeating pattern by strong electrostatic forces. (b) Ionic compounds are hard because their strong bonds resist being stretched. (c) An ionic compound breaks when struck with a hammer because its lattice structure is offset, and like charges repel each other. (d) Ionic compounds conduct electricity ...
Chemistry Vocab for Quiz 12/21 or 12/22 Atom – The smallest
... Chemical change – A change in matter that produces a new substance. Solution – A well mixed mixture. Solubility – A measure of how well a solute can be dissolved at a given temperature. Solvent – The part of the solution present in the largest amount and that dissolves other substances Solute - The ...
... Chemical change – A change in matter that produces a new substance. Solution – A well mixed mixture. Solubility – A measure of how well a solute can be dissolved at a given temperature. Solvent – The part of the solution present in the largest amount and that dissolves other substances Solute - The ...