2012 General Chemistry I
... 2) Placing valence electrons in the hybridized orbitals as pairs (↑↓) or leaving them localized in lone-pair orbitals on individual atoms in the molecule. VSEPR theory is a simplified one : powerful way of predicting the shape of simple molecules. --- does not explain many things including multiple ...
... 2) Placing valence electrons in the hybridized orbitals as pairs (↑↓) or leaving them localized in lone-pair orbitals on individual atoms in the molecule. VSEPR theory is a simplified one : powerful way of predicting the shape of simple molecules. --- does not explain many things including multiple ...
CHEM 120 WEEK 11 LECTURES (INORGANIC WEEK 2) Dr. MD
... Contains only metals, apart from boron. Boron is also the only element which does not form a stable trication (B3+) again will have too high a charge density to be stable. Why do the other elements form tri-cations (M3+ )? Soln. √ Because they have the valence electronic configuration ns2np1 and ...
... Contains only metals, apart from boron. Boron is also the only element which does not form a stable trication (B3+) again will have too high a charge density to be stable. Why do the other elements form tri-cations (M3+ )? Soln. √ Because they have the valence electronic configuration ns2np1 and ...
AP Chemistry
... Composed of molecules, all of which are alike Small! One billionth of a drop of water has 1 trillion molecules!!! ...
... Composed of molecules, all of which are alike Small! One billionth of a drop of water has 1 trillion molecules!!! ...
chemistry 1
... More than 100 elements are known, but only about two dozen are commonly found in living organisms. Elements are represented by one- or two-letter symbols. For example, C stands for carbon, H for hydrogen, Na for sodium, and Hg for mercury (shown). ...
... More than 100 elements are known, but only about two dozen are commonly found in living organisms. Elements are represented by one- or two-letter symbols. For example, C stands for carbon, H for hydrogen, Na for sodium, and Hg for mercury (shown). ...
Second Semester Notes 09-10
... (except in H2O2 and w/ F) **The sum of the oxidation numbers in a compound must equal zero. **The sum of the oxidation number in a PAI is equal to the charge of the ion. **All uncombined elements and diatomics have an oxidation number of zero. ...
... (except in H2O2 and w/ F) **The sum of the oxidation numbers in a compound must equal zero. **The sum of the oxidation number in a PAI is equal to the charge of the ion. **All uncombined elements and diatomics have an oxidation number of zero. ...
Molecular Compound
... • Compare a chemical formula for a molecular compounds with one for an ionic compound. • Discuss the arrangements of ions in crystals. • Define lattice energy and explain its significance. • List and compare the distinctive properties of ionic and molecular compounds. • Write the Lewis structure for ...
... • Compare a chemical formula for a molecular compounds with one for an ionic compound. • Discuss the arrangements of ions in crystals. • Define lattice energy and explain its significance. • List and compare the distinctive properties of ionic and molecular compounds. • Write the Lewis structure for ...
Chapter 2 – Fundamental Building Blocks: Chemistry, Water, and pH
... o The oxygen atom has greater power to attract electrons to itself than do the hydrogen atoms o The term for measuring this kind of pull is electronegativity o The oxygen atom has more electronegativity than do the hydrogen atoms so it tends to pull the shared electrons away from the hydrogen and to ...
... o The oxygen atom has greater power to attract electrons to itself than do the hydrogen atoms o The term for measuring this kind of pull is electronegativity o The oxygen atom has more electronegativity than do the hydrogen atoms so it tends to pull the shared electrons away from the hydrogen and to ...
Atomic Structure - s3.amazonaws.com
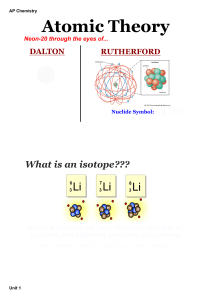
... Early Models of the Atom Dalton’s Atomic Theory (Between 1766-1844) Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. Chemical reactions when atoms are separated, joined, or rearranged. Atoms of one element are neve ...
... Early Models of the Atom Dalton’s Atomic Theory (Between 1766-1844) Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. Chemical reactions when atoms are separated, joined, or rearranged. Atoms of one element are neve ...
Chapter 1-
... • Each p orbital does not just overlap with one adjacent p but overlaps with p orbitals on either side to give a continuous bonding molecular orbital that encompasses all 6 carbons • All 6 p electrons are therefore delocalized over the entire ring and this results in the equivalence of all of the ca ...
... • Each p orbital does not just overlap with one adjacent p but overlaps with p orbitals on either side to give a continuous bonding molecular orbital that encompasses all 6 carbons • All 6 p electrons are therefore delocalized over the entire ring and this results in the equivalence of all of the ca ...
Atoms and Molecules - Library Video Company
... groups of atoms called molecules. Atoms themselves are made of subatomic particles called electrons, protons and neutrons.The nucleus of every atom is made of a certain number of protons and neutrons and orbiting this nucleus are smaller particles called electrons.These particles each have an electr ...
... groups of atoms called molecules. Atoms themselves are made of subatomic particles called electrons, protons and neutrons.The nucleus of every atom is made of a certain number of protons and neutrons and orbiting this nucleus are smaller particles called electrons.These particles each have an electr ...
Atoms and Molecules - Distribution Access
... groups of atoms called molecules. Atoms themselves are made of subatomic particles called electrons, protons and neutrons.The nucleus of every atom is made of a certain number of protons and neutrons and orbiting this nucleus are smaller particles called electrons.These particles each have an electr ...
... groups of atoms called molecules. Atoms themselves are made of subatomic particles called electrons, protons and neutrons.The nucleus of every atom is made of a certain number of protons and neutrons and orbiting this nucleus are smaller particles called electrons.These particles each have an electr ...
Water
... – If the body’s pH level goes to high or too low then cells do not work – Maintaining pH levels is important for homeostasis – Large changes in pH are controlled by substances called buffers ...
... – If the body’s pH level goes to high or too low then cells do not work – Maintaining pH levels is important for homeostasis – Large changes in pH are controlled by substances called buffers ...
Atomic Structure
... • Name and Describe the Parts of an Atom • Identify the Relationships Among the Atomic #, Isotopes, Mass #, & Average Atomic Mass of an Atom. • Calculate the Numbers of Protons, Neutrons, & Electrons of an Atom Given Its Symbol and Mass Number. • Calculate the Average Atomic Mass of an Atom ...
... • Name and Describe the Parts of an Atom • Identify the Relationships Among the Atomic #, Isotopes, Mass #, & Average Atomic Mass of an Atom. • Calculate the Numbers of Protons, Neutrons, & Electrons of an Atom Given Its Symbol and Mass Number. • Calculate the Average Atomic Mass of an Atom ...
Fall Semester Review Packet
... 16. Write the formulas for sodium carbonate, sodium bicarbonate, and calcium phosphate. ...
... 16. Write the formulas for sodium carbonate, sodium bicarbonate, and calcium phosphate. ...
History and Current Status of the Plastics Industry
... » Nylon 6 has 1 dipole + 1 hydrogen bond for every 6 Carbon atoms » Nylon 12 has 1 dipole and 1 hydrogen bond every 12 Carbon atoms – Dipole induces polarity and occurs if » C-Cl single bond (like PVC); C-Fl single bond (Like PTFE); C=O double bond (like Nylon, PET, PU, PC) – Hydrogen bonds induces ...
... » Nylon 6 has 1 dipole + 1 hydrogen bond for every 6 Carbon atoms » Nylon 12 has 1 dipole and 1 hydrogen bond every 12 Carbon atoms – Dipole induces polarity and occurs if » C-Cl single bond (like PVC); C-Fl single bond (Like PTFE); C=O double bond (like Nylon, PET, PU, PC) – Hydrogen bonds induces ...
CHAPTER 2 - Net Start Class
... nucleus--contains the protons and the neutrons; the electrons are located outside the nucleus. Diameter = 10-13 cm. The electrons are located 10-8cm from the nucleus. A mass of nuclear material the size of a pea would weigh 250 million tons! Very dense! proton--positive charge, responsible for the i ...
... nucleus--contains the protons and the neutrons; the electrons are located outside the nucleus. Diameter = 10-13 cm. The electrons are located 10-8cm from the nucleus. A mass of nuclear material the size of a pea would weigh 250 million tons! Very dense! proton--positive charge, responsible for the i ...
Chemistry 11 Review Sheet
... 50. If the following pairs of atoms were to form a bond, which would be the correct method of representing the bond dipole? ...
... 50. If the following pairs of atoms were to form a bond, which would be the correct method of representing the bond dipole? ...