Names and Formulas of Acids 2.8 Naming Inorganic Compounds
... Chemists learned to measure the amounts of elements ...
... Chemists learned to measure the amounts of elements ...
Lecture 9
... Oxidation number is a property of a single atom. We cannot define the oxidation number for a molecule or a polyatomic ion. The sum of oxidation numbers of the atoms in a polyatomic ion or molecule can be calculated. This is not the oxidation number of the molecule or ion. Polyatomic ions have an ov ...
... Oxidation number is a property of a single atom. We cannot define the oxidation number for a molecule or a polyatomic ion. The sum of oxidation numbers of the atoms in a polyatomic ion or molecule can be calculated. This is not the oxidation number of the molecule or ion. Polyatomic ions have an ov ...
chemisty_ass_2
... 8c.(i). Shielding and Screening effect of the inner electrons: Down a group, the shielding of outer electrons by inner electrons overcomes the influence on the increasing nuclear charge, thus the outer electron is shielded from the nucleus by the repelling effect of the inner electrons. Across the g ...
... 8c.(i). Shielding and Screening effect of the inner electrons: Down a group, the shielding of outer electrons by inner electrons overcomes the influence on the increasing nuclear charge, thus the outer electron is shielded from the nucleus by the repelling effect of the inner electrons. Across the g ...
Reactions of Metals and Their Compounds
... …with a difference. I will give you the answer, you have to write the question! For example: Answer = Ms. Lee Question? Who is the most awesome teacher in the world, with beautiful long hair and a wonderful personality. And she is very nice and funny too. ...
... …with a difference. I will give you the answer, you have to write the question! For example: Answer = Ms. Lee Question? Who is the most awesome teacher in the world, with beautiful long hair and a wonderful personality. And she is very nice and funny too. ...
File
... Physical or chemical change? The rain turned to snow… Marty broke a class on the bathroom floor… I burned my bagel! I fried eggs for breakfast… I mixed baking soda and vinegar for science ...
... Physical or chemical change? The rain turned to snow… Marty broke a class on the bathroom floor… I burned my bagel! I fried eggs for breakfast… I mixed baking soda and vinegar for science ...
Chapter 4 Chemical Foundations: Elements, Atoms, and Ions
... Writing Formulas of Compounds • Each element is represented by its symbol. • The number of each type of atom is indicated by a subscript written to the right of the element symbol. • If only one atom is present, do not include a subscript. • If polyatomic groups are present in the molecule, they ar ...
... Writing Formulas of Compounds • Each element is represented by its symbol. • The number of each type of atom is indicated by a subscript written to the right of the element symbol. • If only one atom is present, do not include a subscript. • If polyatomic groups are present in the molecule, they ar ...
Chemistry FINAL: CONTENT Review Packet
... _______________________is made from two or more substances that are physically combined ______________________________ are substances that are made up of only one type of atom _________________________________ is anything that has both mass and volume _____________________________________is a solid, ...
... _______________________is made from two or more substances that are physically combined ______________________________ are substances that are made up of only one type of atom _________________________________ is anything that has both mass and volume _____________________________________is a solid, ...
Chemistry can be defined as the study of the composition, structure
... Chemical Symbols and formulae: It is convenient to use symbols for the atoms of the different elements. An atomic symbol is a one or two letter notation used to represent an atom corresponding to a particular element. Typically the atomic symbol consists of the first letter in capitals from the name ...
... Chemical Symbols and formulae: It is convenient to use symbols for the atoms of the different elements. An atomic symbol is a one or two letter notation used to represent an atom corresponding to a particular element. Typically the atomic symbol consists of the first letter in capitals from the name ...
Ch 11 Review - mvhs
... (c) Butane is non-polar and cannot form hydrogen bonds; 1-propanol is polar and can form hydrogen bonds. 1-propanol can interact with water by both dipole-dipole forces and hydrogen bonds. Butane can interact with water by neither means. Thus, 1-propanol is much more soluble. (d) Acetone molecules a ...
... (c) Butane is non-polar and cannot form hydrogen bonds; 1-propanol is polar and can form hydrogen bonds. 1-propanol can interact with water by both dipole-dipole forces and hydrogen bonds. Butane can interact with water by neither means. Thus, 1-propanol is much more soluble. (d) Acetone molecules a ...
Grade 11 Chemistry Exam Review
... Element X has an electronegativity of 3.0 and element Y. has an electronegativity of 1.0. The most probable type of bond between X and Y is a) pure covalent. b) polar covalent. c) ionic. d) unpredictable. ...
... Element X has an electronegativity of 3.0 and element Y. has an electronegativity of 1.0. The most probable type of bond between X and Y is a) pure covalent. b) polar covalent. c) ionic. d) unpredictable. ...
Chemistry in Biology
... • Compounds are pure substances formed when two or more different elements combine. -Ex: H20, CO2, and C6H12O6 • Compounds are always formed from a specific combination of elements in a fixed ratio. • Compounds cannot be broken down into simpler compounds or elements by physical means. -can be broke ...
... • Compounds are pure substances formed when two or more different elements combine. -Ex: H20, CO2, and C6H12O6 • Compounds are always formed from a specific combination of elements in a fixed ratio. • Compounds cannot be broken down into simpler compounds or elements by physical means. -can be broke ...
Deconstructed HS-PS1-2
... Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.[Clarification Statement: Examples of chemical reactions could include the reaction of ...
... Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.[Clarification Statement: Examples of chemical reactions could include the reaction of ...
Scientific Principles: Chemical Properties
... • Is a major component of food • Is an excellent location for bacterial growth and food spoilage to occur • Can be measured by obtaining the water activity of a food • Must be controlled in foods to aid in food preservation • Is controlled in foods by dehydration, freezing and ...
... • Is a major component of food • Is an excellent location for bacterial growth and food spoilage to occur • Can be measured by obtaining the water activity of a food • Must be controlled in foods to aid in food preservation • Is controlled in foods by dehydration, freezing and ...
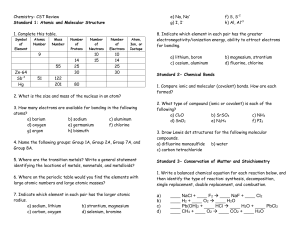
Chemistry- CST Review
... 4. Calculate the molarity of each of the following solutions: a) 0.60 mol of NaCl dissolved in 1.6 L of solution. b) 25.2 g of potassium nitrate, KNO3, in enough water to make 150.0 mL of solution. 5. Calculate the number of grams of solute needed to prepare each of the following solutions: a) 4500. ...
... 4. Calculate the molarity of each of the following solutions: a) 0.60 mol of NaCl dissolved in 1.6 L of solution. b) 25.2 g of potassium nitrate, KNO3, in enough water to make 150.0 mL of solution. 5. Calculate the number of grams of solute needed to prepare each of the following solutions: a) 4500. ...
Summary of Chapter 2
... Common names are traditional names for substances (e.g., water, ammonia). Systematic names are based on a systematic set of rules. • Divided into organic compounds (those containing C, usually in combination with H, O, N, or S) and inorganic compounds (all other compounds). ...
... Common names are traditional names for substances (e.g., water, ammonia). Systematic names are based on a systematic set of rules. • Divided into organic compounds (those containing C, usually in combination with H, O, N, or S) and inorganic compounds (all other compounds). ...
Ionic Equations
... If product is a gas that has a low solubility in water, reaction in solution is driven to produce the gas Tums relief Any carbonate with an acid NaHCO3(s) + HCl(aq) = NaCl(aq) + H2O(l) + CO2(g) ...
... If product is a gas that has a low solubility in water, reaction in solution is driven to produce the gas Tums relief Any carbonate with an acid NaHCO3(s) + HCl(aq) = NaCl(aq) + H2O(l) + CO2(g) ...
Chemistry Unit Review
... 14.Give chemical formulas for the compounds found above. a. b. c. d. e. f. g. h. i. j. 15. Write word equations for the following. a. A reaction between calcium and oxygen produces calcium oxide. ...
... 14.Give chemical formulas for the compounds found above. a. b. c. d. e. f. g. h. i. j. 15. Write word equations for the following. a. A reaction between calcium and oxygen produces calcium oxide. ...
Atom - Images
... determines atom’s chemical properties participate in chemical bonding Every atom has between one and eight ...
... determines atom’s chemical properties participate in chemical bonding Every atom has between one and eight ...
Atom
... determines atom’s chemical properties participate in chemical bonding Every atom has between one and eight ...
... determines atom’s chemical properties participate in chemical bonding Every atom has between one and eight ...