Chemistry I Exams and Keys 2014 Season
... A. They are both isomers of one another. B. They both represent the same compound. C. They have different empirical formulas. D. They are called isotopes of the same substance. E. They are called allotropes of the same substance. ...
... A. They are both isomers of one another. B. They both represent the same compound. C. They have different empirical formulas. D. They are called isotopes of the same substance. E. They are called allotropes of the same substance. ...
1.What is the overall charge of an ion that has 12 protons
... units. This atomic mass represents the A) total mass of all the protons and neutrons in an atom of Ti B) total mass of all the protons, neutrons, and electrons in an atom of Ti C) weighted average mass of the most abundant isotope of Ti D) weighted average mass of all the naturally occurring isotope ...
... units. This atomic mass represents the A) total mass of all the protons and neutrons in an atom of Ti B) total mass of all the protons, neutrons, and electrons in an atom of Ti C) weighted average mass of the most abundant isotope of Ti D) weighted average mass of all the naturally occurring isotope ...
Physical Properties
... Pure Substances • A pure substance has well defined physical and chemical properties. • Pure substances can be classified as elements or compounds. • Compounds can be further reduced into two or more elements. • Elements consist of only one type of atom. They cannot be decomposed or further simplifi ...
... Pure Substances • A pure substance has well defined physical and chemical properties. • Pure substances can be classified as elements or compounds. • Compounds can be further reduced into two or more elements. • Elements consist of only one type of atom. They cannot be decomposed or further simplifi ...
Lecture 21 – Cations, Anions and Hydrolysis in
... Hydrolysis involves further chemical reaction of hydrated ions with the solvent (water). Metal ions in aqueous solution behave as Lewis acids. The positive charge on the metal ion draws electron density from the O-H bond in the water. This increases the bond's polarity making it easier to break. Whe ...
... Hydrolysis involves further chemical reaction of hydrated ions with the solvent (water). Metal ions in aqueous solution behave as Lewis acids. The positive charge on the metal ion draws electron density from the O-H bond in the water. This increases the bond's polarity making it easier to break. Whe ...
Atomic Theory and Bonding
... If the valence electrons can combine to form a low-energy bond, a compound is formed. Each atom in the compound attempts to have the stable number of valence electrons as the nearest noble gas. Metals may lose electrons and non-metals gain electrons, (ionic bond) OR Atoms may share electrons ...
... If the valence electrons can combine to form a low-energy bond, a compound is formed. Each atom in the compound attempts to have the stable number of valence electrons as the nearest noble gas. Metals may lose electrons and non-metals gain electrons, (ionic bond) OR Atoms may share electrons ...
Notes for Matter Packet- Balancing equations (PDF
... 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in o ...
... 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in o ...
Atoms and Molecules - Gulfport School District
... •These are important bonds in the human body. •Hydrogen bonds play an important role in the shape of complex molecules such as proteins and ...
... •These are important bonds in the human body. •Hydrogen bonds play an important role in the shape of complex molecules such as proteins and ...
Molecules and Molecular Compounds
... 2) change the –ide ending on the second element to –ic. hydrogen chloride + –ic acid → hydrochloric acid ...
... 2) change the –ide ending on the second element to –ic. hydrogen chloride + –ic acid → hydrochloric acid ...
File
... • Octet Rule: atoms will bond with other atoms to gain, lose or share electrons in order to achieve a full outer shell (i.e. 8) • Ion = charged atom • Ionic bond: is formed between positive ions and negative ions which are attracted to each other and bond together. • Most ionic compounds contain a ...
... • Octet Rule: atoms will bond with other atoms to gain, lose or share electrons in order to achieve a full outer shell (i.e. 8) • Ion = charged atom • Ionic bond: is formed between positive ions and negative ions which are attracted to each other and bond together. • Most ionic compounds contain a ...
A Thumbnail Review of Regents Chemistry
... Most metallic element = Fr = loses electrons most readily Leas metallic element = F = gains electrons most readily Allotropes = different forms of same elements with different structures and different properties Elements in same group = same # of valence electrons = similar chemical and physical pro ...
... Most metallic element = Fr = loses electrons most readily Leas metallic element = F = gains electrons most readily Allotropes = different forms of same elements with different structures and different properties Elements in same group = same # of valence electrons = similar chemical and physical pro ...
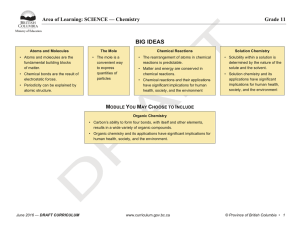
BIG IDEAS - BC Curriculum - Province of British Columbia
... • Carbon’s ability to form four bonds, with itself and other elements, results in a wide variety of organic compounds. • Organic chemistry and its applications have significant implications for human health, society, and the environment. ...
... • Carbon’s ability to form four bonds, with itself and other elements, results in a wide variety of organic compounds. • Organic chemistry and its applications have significant implications for human health, society, and the environment. ...
Chemical Foundations: Elements, Atoms, and Ions
... • Arranged according to increasing atomic number (number of protons) • Horizontal Rows – • Vertical Columns – • This arrangement is based on chemical similarities that exist in the vertical columns (groups). These groups are referred to as • This system of arrangement was 1st proposed by Dmitri Mend ...
... • Arranged according to increasing atomic number (number of protons) • Horizontal Rows – • Vertical Columns – • This arrangement is based on chemical similarities that exist in the vertical columns (groups). These groups are referred to as • This system of arrangement was 1st proposed by Dmitri Mend ...
Ch. 8 Notes (Chemical Reactions) Teacher Relearn
... Step 1-- use your ion sheet and find the ions and their charges. Step 2-- “Cross the charges” if they don’t balance out. Step 3-- Use parentheses around polyatomic ion “chunks”. Practice Problems: Write the formula for each ionic compound. ...
... Step 1-- use your ion sheet and find the ions and their charges. Step 2-- “Cross the charges” if they don’t balance out. Step 3-- Use parentheses around polyatomic ion “chunks”. Practice Problems: Write the formula for each ionic compound. ...
The Modern View of Atomic Structure
... Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. Average mass is calculated from the isotopes of an element weighted by their relative abundances. ...
... Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. Average mass is calculated from the isotopes of an element weighted by their relative abundances. ...
Chapter 2
... number and kinds of atom. 4. Chemical reactions are rearrangement of atoms. Atoms are not created or destroyed. ...
... number and kinds of atom. 4. Chemical reactions are rearrangement of atoms. Atoms are not created or destroyed. ...
Atomic Structure and Notes_AISD ppt
... they fired Helium nuclei at a piece of gold foil which was only a few atoms thick. they found that although most of them passed through. About 1 in 10,000 hit ...
... they fired Helium nuclei at a piece of gold foil which was only a few atoms thick. they found that although most of them passed through. About 1 in 10,000 hit ...
ch 18 review filled in
... How many electron levels does carbon have? Two (but only one is full) How many full electron levels does Krypton have? Four (Krypton completes level four) ...
... How many electron levels does carbon have? Two (but only one is full) How many full electron levels does Krypton have? Four (Krypton completes level four) ...
Essential Standard: 8.P.1 Understand the properties of matter and
... occur when matter interacts in an open and closed container. Clarifying Objective: 8.P.1.1 Classify matter as elements, compounds, or mixtures based on how the atoms are packed together in arrangements. Students need to know: the structure of the atom: o ...
... occur when matter interacts in an open and closed container. Clarifying Objective: 8.P.1.1 Classify matter as elements, compounds, or mixtures based on how the atoms are packed together in arrangements. Students need to know: the structure of the atom: o ...
Definitions for Concept Map
... Holes are free space within a packing structure; usually filled with smaller cations for ionic solids. → Octahedral hole is coordinated by six anions. → Tetrahedral hole is coordinated by four anions. Formula Unit of Ionic Compound is the empirical formula of an ionic or covalent network solid; ...
... Holes are free space within a packing structure; usually filled with smaller cations for ionic solids. → Octahedral hole is coordinated by six anions. → Tetrahedral hole is coordinated by four anions. Formula Unit of Ionic Compound is the empirical formula of an ionic or covalent network solid; ...
Endothermic And Exothermic Reactions
... Endothermic reactions A chemical reaction that absorbs energy from its surroundings. More energy is required to break the bonds in the reactants than is released by the formation of bonds in the products. In these reactions, heat is shown as one This is a typical graph of an of the reactants endoth ...
... Endothermic reactions A chemical reaction that absorbs energy from its surroundings. More energy is required to break the bonds in the reactants than is released by the formation of bonds in the products. In these reactions, heat is shown as one This is a typical graph of an of the reactants endoth ...