DELTAHPP
... Calculate standard enthalpy changes using bond enthalpy values Calculate standard enthalpy changes using enthalpies of formation and combustion Know simple calorimetry methods for measuring enthalpy changes Calculate enthalpy changes from calorimetry measurements ...
... Calculate standard enthalpy changes using bond enthalpy values Calculate standard enthalpy changes using enthalpies of formation and combustion Know simple calorimetry methods for measuring enthalpy changes Calculate enthalpy changes from calorimetry measurements ...
2013 - SQA
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. SECTION A—Questions 1–30 (30 marks) Instructions for completion of Section A are given on page two. For this section of the examination you must use an HB pencil. SECTION B (70 marks) 1 All questions should be attempted ...
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. SECTION A—Questions 1–30 (30 marks) Instructions for completion of Section A are given on page two. For this section of the examination you must use an HB pencil. SECTION B (70 marks) 1 All questions should be attempted ...
deltahpps
... Calculate standard enthalpy changes using bond enthalpy values Calculate standard enthalpy changes using enthalpies of formation and combustion Know simple calorimetry methods for measuring enthalpy changes Calculate enthalpy changes from calorimetry measurements ...
... Calculate standard enthalpy changes using bond enthalpy values Calculate standard enthalpy changes using enthalpies of formation and combustion Know simple calorimetry methods for measuring enthalpy changes Calculate enthalpy changes from calorimetry measurements ...
Chapter One Hemilabile Ligands in Transition
... In this very simple example, the enthalpy difference is within experimental error indicating the chelate effect can be traced entirely to the entropy difference between the change of ligand numbers.5 The main cause of the large entropy increase is related to the net number of unbound molecules or li ...
... In this very simple example, the enthalpy difference is within experimental error indicating the chelate effect can be traced entirely to the entropy difference between the change of ligand numbers.5 The main cause of the large entropy increase is related to the net number of unbound molecules or li ...
content - Thesis Scientist
... P or As, the dopant atom forms four covalent bonds like a Si or Ge atom but the fifth electron, not used in bonding, becomes delocalised and contribute its share towards electrical conduction. Thus silicon or germanium doped with P or As is called n-type semiconductor, n indicative of negative, sinc ...
... P or As, the dopant atom forms four covalent bonds like a Si or Ge atom but the fifth electron, not used in bonding, becomes delocalised and contribute its share towards electrical conduction. Thus silicon or germanium doped with P or As is called n-type semiconductor, n indicative of negative, sinc ...
Chapter 4: Reactions in Aqueous Solution
... 44. Which choice gives the correct oxidation numbers for all three elements in Rb2SO3 in the order that the elements are shown in the formula? A) –2, +6, –2 B) –1, +4, –3 C) +2, +4, –2 D) +1, +4, –2 E) +1, +6, –6 Ans: D Category: Medium Section: 4.4 45. Which choice gives the correct oxidation numbe ...
... 44. Which choice gives the correct oxidation numbers for all three elements in Rb2SO3 in the order that the elements are shown in the formula? A) –2, +6, –2 B) –1, +4, –3 C) +2, +4, –2 D) +1, +4, –2 E) +1, +6, –6 Ans: D Category: Medium Section: 4.4 45. Which choice gives the correct oxidation numbe ...
Chapter 3: Mass Relationships in Chemical
... 58. A mass spectrometer works by ionizing atoms or molecules, and then accelerating them past oppositely charged plates. The mass is obtained by A) measuring the force of impact on a detecting screen, and then calculating the mass using force = mass acceleration. B) suspending the ions in an appli ...
... 58. A mass spectrometer works by ionizing atoms or molecules, and then accelerating them past oppositely charged plates. The mass is obtained by A) measuring the force of impact on a detecting screen, and then calculating the mass using force = mass acceleration. B) suspending the ions in an appli ...
Chemical Quantities
... that chemical changes are actually rearrangements of atom groupings that can be described by chemical equations. These chemical equations tell us the identities (formulas) of the reactants and products and also show how much of each reactant and product participates in the reaction. The numbers (coe ...
... that chemical changes are actually rearrangements of atom groupings that can be described by chemical equations. These chemical equations tell us the identities (formulas) of the reactants and products and also show how much of each reactant and product participates in the reaction. The numbers (coe ...
HOTS Worksheet
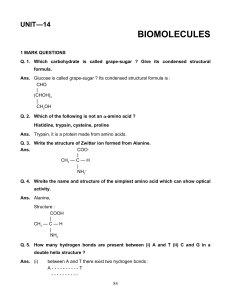
... Ans. (i) Glucose does not give Schiff‘s Test although it contains aldehyde group. (ii) Glucose does not form crystaline product with NaHSO3. Q. 6. B-complex is an often prescribed Vitamin. What is complex about it ? What is its usefulness ? Ans. It is a type of Vitamin which contains B1, B2, B6 and ...
... Ans. (i) Glucose does not give Schiff‘s Test although it contains aldehyde group. (ii) Glucose does not form crystaline product with NaHSO3. Q. 6. B-complex is an often prescribed Vitamin. What is complex about it ? What is its usefulness ? Ans. It is a type of Vitamin which contains B1, B2, B6 and ...
Mole-mole factor
... • Stoichiometry is the study of the quantitative relationships among reactants and products in a chemical reaction • These chemical calculations can be used to determine the amount of one reactant needed to completely react with another • Or, to determine the amount of reactant needed to produce a d ...
... • Stoichiometry is the study of the quantitative relationships among reactants and products in a chemical reaction • These chemical calculations can be used to determine the amount of one reactant needed to completely react with another • Or, to determine the amount of reactant needed to produce a d ...
Modern Chemistry
... the answer as 0.571429. a. Is the setup for calculating density correct? b. How many significant figures should the answer contain? 4. It was shown in the text that in a value such as 4000 g, the precision of the number is uncertain. The zeros may or may not be significant. a. Suppose that the mass ...
... the answer as 0.571429. a. Is the setup for calculating density correct? b. How many significant figures should the answer contain? 4. It was shown in the text that in a value such as 4000 g, the precision of the number is uncertain. The zeros may or may not be significant. a. Suppose that the mass ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.